Salaam (May God Bless You). Radioactivity is the conversion of small amount of masses into energy. The radioactivity was first discovered by a French scientist, Henri Becquerel in 1896. He was experimenting with a mineral which glows in the dark. He wrapped a black paper around the mineral rock and placed it on plate in a drawer. After 24 hours, he checked the mineral and found that there was a mist on the plate which is normally seen when exposed to light. This was unusual as the mineral was wrapped in black paper. He had discovered radioactivity.
Three Types of Radiations
Since the discovery of radioactivity much work has been done by various scientists, to discover the properties of radiation. The radiations were named after the first
three Greek letters, alpha (α), beta(β) and gamma (γ). These three radiations are ionising radiations, because they impact on molecules, this impact causes the electron of the molecules being pushed out. This ionizes the molecules. This is why the radiations are dangerous to humans, they may enter the human body, ionising  the cells and causing mutation which may cause a tumor or trigger cancer.
the cells and causing mutation which may cause a tumor or trigger cancer.
Alpha particles are positive particles of helium nucleus. These particles are large and have a great amount of kinetic energy. This particle has two protons and two neutrons. Since it has a greater mass it does not travel far as it collides with air molecules, ionizes them and are soon absorbed. The alpha radiation is harmless to humans as it is not very penetrating but if enters the body may cause adverse affects. The alpha particles can be stopped by a sheet of paper.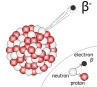
The Beta Particles are negatively charged particles or more accurately electrons. The electrons have a small mass which is why the electrons have less kinetic energy. The electrons can go further than alpha radiation and can stopped by a few millimeters of aluminium. These are very dangerous to humans as it can penetrate the skin.
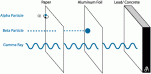 Gamma radiation are waves which are part of an electromagnetic spectrum. These waves have a high frequency and very low wavelength. These waves are neither positive nor negative. Several centimeters of dense metal is required. It is very penetrating to the human body which is why it is most dangerous.
Gamma radiation are waves which are part of an electromagnetic spectrum. These waves have a high frequency and very low wavelength. These waves are neither positive nor negative. Several centimeters of dense metal is required. It is very penetrating to the human body which is why it is most dangerous.
Deflection in electrical and magnetic fields
The radioactive waves are either positive, neutral or negatively charged, which is why these radiations can be passed through magnetic fields and electric fields. In 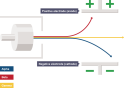 an electric field, the alpha radiation is deflected towards the negative field because it is attracted towards it but is deflected by the positive field. The beta particles are deflected by the negative field and attracted towards the positive field, since the beta particles are very small in mass compared to alpha particles they are pushed in greater amount than alpha particles. Gamma radiation is not deflected but passes straight through both the magnetic field and the electric field.
an electric field, the alpha radiation is deflected towards the negative field because it is attracted towards it but is deflected by the positive field. The beta particles are deflected by the negative field and attracted towards the positive field, since the beta particles are very small in mass compared to alpha particles they are pushed in greater amount than alpha particles. Gamma radiation is not deflected but passes straight through both the magnetic field and the electric field.
Detecting Radiation
Radiation at work places such as a hospital is detected by the radiologists which wear a special badge with photographic film in it. The radiation reacts with the photographic film which is sealed from light. These photographic films help detect the amount of radiation the wearer is exposed to and is replaced regularly.
it. The radiation reacts with the photographic film which is sealed from light. These photographic films help detect the amount of radiation the wearer is exposed to and is replaced regularly.
For scientific purposes, cloud chambers are used to detect the way the radiation particles move about. A cloud chamber has moist air which condenses on to the ionised air.
purposes, cloud chambers are used to detect the way the radiation particles move about. A cloud chamber has moist air which condenses on to the ionised air.
Geiger-Muller Tube is common device used for detecting radiation. The tube is a metal casing containing a gas at low pressure with a thin mica window in its end. The tube contains a wire with positive voltage. When the radiation 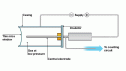 enters the casing, the radiation ionizes the air which then touches the wire passing on a current. An electronic pulse counter is connected with the tube to count the level of radiation.
enters the casing, the radiation ionizes the air which then touches the wire passing on a current. An electronic pulse counter is connected with the tube to count the level of radiation.
Half-Life
Radioactive isotopes are unstable elements which tend to decay over time. The amount of time it takes for the isotopes to decay almost half of its mass is called its 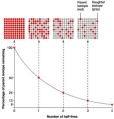 half-life. The formula for half-life is t1/2. If radioactive decay occurs, the isotope then emits radiation throughout. The fall of its activity can be obtained by measuring its activity at different times. The count rate falls as the element decays and so the amount of time it takes for the count rate to fall to a value half to the initial value is its half-life.
half-life. The formula for half-life is t1/2. If radioactive decay occurs, the isotope then emits radiation throughout. The fall of its activity can be obtained by measuring its activity at different times. The count rate falls as the element decays and so the amount of time it takes for the count rate to fall to a value half to the initial value is its half-life.
Background Radiation
When measuring radiation of an element, we must first measure the background radiation as the ground also emits radiation which adds up to the count rate measured. The radiation is most commonly the result of the gas radon being released from beneath the Earth. To measure the count rate to an accuracy, first measure the count rate of the background radiation and subtract it from the count rate of the intended object.
the count rate measured. The radiation is most commonly the result of the gas radon being released from beneath the Earth. To measure the count rate to an accuracy, first measure the count rate of the background radiation and subtract it from the count rate of the intended object.
Carbon-14 Dating
Carbon is an element found in almost all living things and non living things. It also has many isotopes such as carbon-12 and carbon-14. These two isotopes are constant in the environment and are constantly cycled through the carbon cycle. The carbon 14 is a radioactive isotope which tends to decay over a period of 5730 years, and so is useful for dating of dead materials or old artifacts. The carbon locks in to an organism once the organism dies, storing the carbon. Scientists burn a sample of the carbon taken from the dead organism, so that the carbon combines with oxygen to form carbon dioxide, the carbon-12 dioxide mixture and carbon-14 dioxide mixture is measured and compared to find out the date the organism died.
For example a sample od dead organism is burned to find that the concentration of carbon-14 dioxide is 3.125% its initial value, we can find out how old the organism or object is by counting its half-life: 3.125% is the same as 1/32, we must count the half life to 1/32, 1→1/2→1/4→1/8→1/16→1/32, it has lived 5 times the half-lives of carbon-14, then we multiply the number with the half life of carbon 14; 5 x 5730 = 28 650 years old.
