Salaam (May God Bless You). Metals are materials which are found on the Earth’s surface which is a very valuable material. Metals are sorted in to the transition elements of the periodic table and the Group I and Group II metals. The Group I and II metals are soft and are easily broken but the transition elements are very hard and ductile. They can be beaten into thin sheets. The atomic structure of the metals is as such that the metals have cations in its structure and are surrounded by free electrons. These free electrons are the cause of the thermal and electrical property of the metals. All metals are good thermal and electrical conductors. Metal cations have similar shape and size which is why they are easy to pull over each other making them ductile. This only involves the transition elements.
Extraction of metals
Metals have existed as precious materials even a thousand yeas ago in the form gold and silver. These metals are least reactive and are found at the bottom of the reactivity series. These are found in ores which can simply be heated to extract it. The metals above gold and silver in the reactivity series include copper, hydrogen lead, and mercury which can be extracted by more intense heat in air. Tin, iron and zinc are extracted by heating them and these form oxides which are then reduced using reducing agents. The last group is very reactive and thus are extracted by a means of an electric charge which is why they remained isolated until electricity was first discovered. The metals were extracted when electrons were passed through them and the cations receive the electrons, the cathode is reduced while the corresponding anions lose electrons and are oxidised at the anode.
Alloys
Alloys are mixtures of metals and/or non-metals. These are rather valuable material more than the pure metal as these have completely different properties from the  initial reactants. The reason for such a difference in physical properties is that the all elements have atoms which are of different sizes which is why when two metals or non metals combine the particles are forced into shape, but they cannot be gain the same properties as before such as an alloy of copper and zinc cannot be malleable and ductile. This is because the zinc atom is large in size than copper atom.
initial reactants. The reason for such a difference in physical properties is that the all elements have atoms which are of different sizes which is why when two metals or non metals combine the particles are forced into shape, but they cannot be gain the same properties as before such as an alloy of copper and zinc cannot be malleable and ductile. This is because the zinc atom is large in size than copper atom.
Reactivity Series
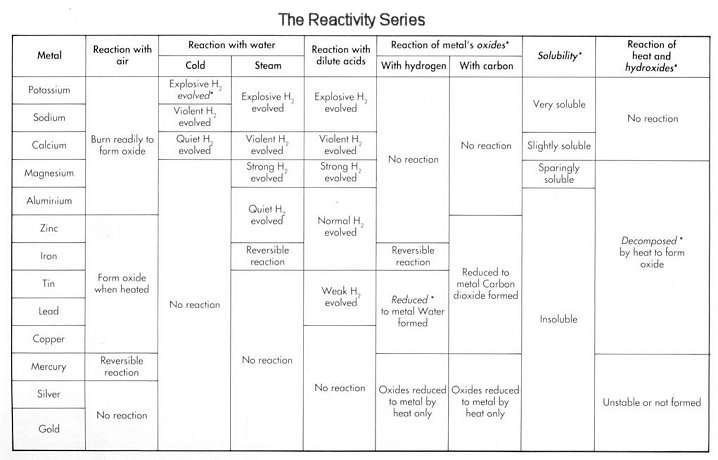 The reactivity series is a group of metals arranged according to their reactivity towards various materials with potassium being the most reactive and gold being the least reactive. This series also have hydrogen and carbon in it. The Group I and II are most reactive and then aluminium and then the transition elements.
The reactivity series is a group of metals arranged according to their reactivity towards various materials with potassium being the most reactive and gold being the least reactive. This series also have hydrogen and carbon in it. The Group I and II are most reactive and then aluminium and then the transition elements.
Displacement Reactions
Displacement reactions are reactions between metals, one more reactive than the other. The most reactive metal will displace the electrons from the least reactive one not the other way around.
Thermal stability of metals
Metals may decompose in heat. Group I metals are most stable in high heat except for lithium, while Group II metals decompose in high heat to produce corresponding metal oxides.. The transition metals decompose readily in fairly low heat to form corresponding oxides.
