Salaam (May God Bless You). The iron and aluminium are two of the most important metals used today. Iron is produced in factories inside a blast furnace. The furnace has various pipes leading to it which are called tuyeres, these emit hot air in to raise the 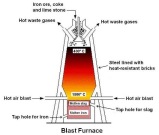 temperature. The hot air goes out through the pipes on the top of the furnace leading out. The upper hole of the furnace is where the products; coke, iron ore and limestone are added. The gases heat up the top of the furnace to 900°C and the lower part is heated to 2400°C. The carbon content in the coke reacts with the hot air in the furnace to produce carbon dioxide and carbon monoxide. The limestone is useful for helping protect
temperature. The hot air goes out through the pipes on the top of the furnace leading out. The upper hole of the furnace is where the products; coke, iron ore and limestone are added. The gases heat up the top of the furnace to 900°C and the lower part is heated to 2400°C. The carbon content in the coke reacts with the hot air in the furnace to produce carbon dioxide and carbon monoxide. The limestone is useful for helping protect

the lining of the furnace making its lifespan longer. The carbon monoxide acts as
the main reducing agent which produces pure iron called pig iron by reacting with the iron oxide ore. The pig iron is drawn off of the pipes while the slag built on top of the pig iron by the reaction between the limestone and carbon dioxide is drawn off from another end to be used as construction material. The carbon monoxide is also produced by this reaction.
Production of aluminium
Aluminium is one of the most reactive metals there is which is why it needs to be electrolyzed to be extracted from its bauxite ore. The aluminium production is done by electrolysis in Hall Heroult process. In this process pure aluminium is formed. The process is a rather difficult one as, aluminium being most reactive towards oxygen rapidly forms oxide layers on it till at least 3 nm deep. These layers are the reason aluminium can be seen as rather inert at first sight. The aluminium is useful for making alloys which are used to make aircraft vehicles. Aluminium is very soft and has low density.
Rust
Metals react with water and air to form oxides which weaken the metal and  reduce its value. The corrosion of metals are called rust. It can be prevented by covering the metal with paints, plastic or even metals which are above the metallic material used in the reactivity series. The galvanization is the process in which iron is covered with zinc to act as protection. If the layer is damaged and water or air pass through, the zinc acts as the anode and the iron as the cathode which keeps the iron protected even after damage. Tinning is the same process, only difference is that iron is protected by tin which is lower than iron in reactivity series. This makes the iron the anode and tin the cathode once damaged making the rusting process faster.
reduce its value. The corrosion of metals are called rust. It can be prevented by covering the metal with paints, plastic or even metals which are above the metallic material used in the reactivity series. The galvanization is the process in which iron is covered with zinc to act as protection. If the layer is damaged and water or air pass through, the zinc acts as the anode and the iron as the cathode which keeps the iron protected even after damage. Tinning is the same process, only difference is that iron is protected by tin which is lower than iron in reactivity series. This makes the iron the anode and tin the cathode once damaged making the rusting process faster.
