Chromatography
Chromatography is the process of using a filter paper or any other absorbent paper to separate pigments. A filter paper’s bottom part is marked with pencil where an ink is stained just enough to concentrate its color on to the paper. The solvent is poured in a petri dish. The solvent might be water but ethanol is preferred due to its high rate of solubility. The paper is than placed vertically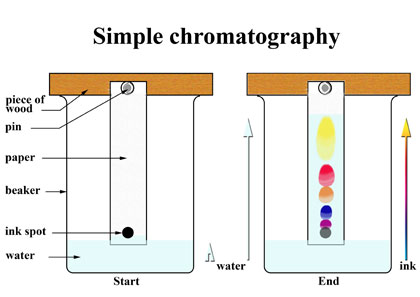 upwards in the dish. The solvent should not touch the ink as it will dissolve in the solvent. As the solvent moves up the paper, the different pigments will get dissolved with the solvent and move up the chromatography paper. As the solvent reaches the top of the paper the pigments of the ink will stop at a certain point. This is a good way of showing the amount of pigments in an ink. The separated pigments will then be called a chromatogram. You will need to measure the Rf value by the equation;
upwards in the dish. The solvent should not touch the ink as it will dissolve in the solvent. As the solvent moves up the paper, the different pigments will get dissolved with the solvent and move up the chromatography paper. As the solvent reaches the top of the paper the pigments of the ink will stop at a certain point. This is a good way of showing the amount of pigments in an ink. The separated pigments will then be called a chromatogram. You will need to measure the Rf value by the equation;
Rf = distance traveled by substance ÷ distance traveled by solvent
Identification of Ions
Ions are charged particles. They get positively charged when they lose electron but when they gain electron they get negatively charged. A positively charged ions are called cations and the negatively charged ions are called anions.
Cations
Cations can be discovered by using sodium hydroxide and aqueous ammonia solution.
- Ammonium ion – In a solution containing ammonium ions add dilute sodium hydroxide solution. This reaction will produce ammonia gas which has a pungent smell. This turns the moist red litmus paper blue.
- Copper (II) ion – In a solution containing copper (II) ions add dilute sodium hydroxide. This reaction will produce a light blue precipitate of copper (II) hydroxide, which will be insoluble in excess sodium hydroxide.
Aqueous ammonia can be added to form a light blue precipitate which will be soluble in excess aqueous ammonia to produce dark blue precipitate.
- Iron (II) ion – To a solution containing Iron (II) ion add sodium hydroxide or aqueous ammonia, which will produce a dirty green precipitate of iron (II) hydroxide, which is insoluble in excess of either of the reagents.
- Iron (III) ions – In such solutions add sodium hydroxide or aqueous ammonia, this reaction will produce red brown precipitate. The solution will be insoluble in excess of any of the reagents.
- Calcium ion – Add sodium hydroxide to such solutions and a white precipitate is formed. Aqueous ammonia is not used here.
- Aluminum ion – Add sodium hydroxide or aqueous ammonia. The reaction will produce a white precipitate. But the test with the sodium hydroxide leads to the solution becoming colorless in excess sodium hydroxide.
- Zinc Ion – Add sodium hydroxide or aqueous ammonia . The reaction gives off white precipitate. The test with aqueous ammonia produces a soluble precipitate which causes the solution to go colorless.
Anions
Anions use a variety of different reagents.
- Carbonate ion – A solution containing carbonate ions is mixed with hydrochloric acid which gives off carbon dioxide. In the presence of lime water the carbon dioxide gets absorbed by it causing it to turn milky or cloudy.
- Chloride ion – Mix the following solution containing chloride ions with a few drops of nitric acid along with silver nitrate solution. A dense white precipitate is formed. This darkens in the presence of sunlight.
- Iodide ion – Add the few drops of nitric acid along with silver nitrate solution to produce bright yellow precipitate.
- Nitrate ions – Add sodium hydroxide to the solution containing nitrate ions along with aluminum powder. This reaction gives off ammonia gas which has a pungent smell and turns moist red litmus paper blue.
- Sulfate ions – Add few drops of nitric acid along with barium nitrate solution. This gives off a white precipitate.
For chloride ions, iodide ions and sulfate ions nitric acid is used to remove carbonate ions which gives off carbon dioxide which causes effervescing of the solution.
In the nitrate test the aluminum powder reduces the nitrate ions to ammonium ions which causes it to give off ammonia gas.
Identification of gases
Ammonia
Ammonia has the characteristic of a pungent smell. The ammonia gas is soluble in water and turns the water alkaline. This is why a red litmus paper is used moist. The ammonia makes the water alkaline causing the litmus paper to go blue.
Carbon dioxide
In case of carbon dioxide lime water is used. The lime water absorbs the carbon dioxide gas and turns milky or cloudy.
Chlorine
Chlorine mixes with water to form an acid. This is then tested with the blue litmus paper. The blue litmus paper is turned red but soon it looses its color as the chlorine also produces bleach in the reaction.
Hydrogen
Hydrogen is highly flammable. It is less dense than air and so is collected by upward delivery method in an inverted tube. In the presence of flame, the gas in the tube sparks and explodes creating “pop” sound.
Oxygen
This gas supports combustion and causes more rise in fire than air itself. A glowing splint is placed over a tube filled with oxygen gas. The gas will cause the glowing splint to catch fire.
Sulfur dioxide
Sulfur dioxide mixes with water to make an acid which can then be tested with a blue litmus paper which would then turn red. But more efficient method is by mixing the sulfur dioxide in acidic potassium manganet (VII). The purple precipitate will soon turn colorless.
Water
For water cobalt chloride paper is used. Water turns cobalt chloride from blue to pink. Water also turns anhydrous copper (II) sulfate from white to blue.
