Pressure in Solids
All objects are pulled towards the Earth’s surface by gravity. This pulling force is called weight. The weight of an object is constant but it will not always exert same pressure. The pressure will only be exerted by the part of the object touching the ground. Such as in normal shoes the pressure is exerted on the ground through the shoes. The amount of area touching the ground is not big enough so greater pressure is exerted or you can say that the weight is spread out over the  area of the boots touching the ground. The snow shoes which have a larger area touching the snow causes the exertion of low amount of pressure.
area of the boots touching the ground. The snow shoes which have a larger area touching the snow causes the exertion of low amount of pressure.
This can be indicating by smoothing out sand on a tray and place a brick on the sand. Place the brick on the sand by the side which has a smaller width or smaller area. Place another brick but this time place it by its side which has more area. Let the brick exert its own pressure and then pull it out. Notice which side had created its mark in more depth. It should be the one with smaller area.
If you press a nail on to a board, the nail will exert great pressure on the board as it has a very small area touching the ground. The same is the case in stiletto high heels.
This equation is used to find out the pressure of an object:
pressure = Force÷Area ; p=F/A
It is measured in Pascals and is expressed by Newton per square meter; N/m^2. When surface in contact with the ground is small then it will be expressed by N/cm^2
Pressure In Liquids
Liquids take on the shape of its container so the pressure exerted by the liquid is dependent on the height of the liquid and the density of the liquid. Pressure in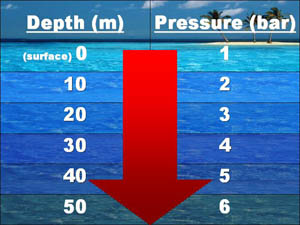 liquid is calculated by the equation:
liquid is calculated by the equation:
p=ρgh ; pressure in liquids = density x gravity x height
It is expressed by Kg/m^3 or Kilogram per cubic meter. It is also expressed as gram per cubic centimeter or g/cm^3.
The pressure on the surface of water is 1 atmosphere due to the weight of air in the atmosphere. The pressure increases every 10 meter in depth.
The pressure in liquids vary if they have different densities such as mercury has greater density then water and so will exert 13.59 times greater pressure than water. This difference in pressure can be found out by a using a U tube. In a U tube add water and oil in the following way as shown in picture. Measure the  length of the oil and the water in the opposite of the oil and use their densities in the equation:
length of the oil and the water in the opposite of the oil and use their densities in the equation:
ρ x g x h = ρ x g x h ; density x gravity x height = density x gravity height
Take one equation on the either side of the equal-to sign as the equation for oil and the other for water. You can use this equation for finding out the density of the oil.
Pressure in Gas
The particles of gas are in a random state of motion and are distant from each other. They also collide with each other and exert a force on each other to move away from each other. If the air is contained in a jar the particles of the gas will collide with the walls of the jar. This forms a pressure. This can be tested by a manometer. The manometer is just like a U tube with a liquid in it. Air is blown into the manometer from one side and the pressure by the air particles causes the liquid to move down the tube on the side where air is blown but rises on the other side of the manometer.
are distant from each other. They also collide with each other and exert a force on each other to move away from each other. If the air is contained in a jar the particles of the gas will collide with the walls of the jar. This forms a pressure. This can be tested by a manometer. The manometer is just like a U tube with a liquid in it. Air is blown into the manometer from one side and the pressure by the air particles causes the liquid to move down the tube on the side where air is blown but rises on the other side of the manometer.
Atmospheric pressure
Air is a homologous mixture of gases which is composed of different gases. These gases exert a pressure on the surface of the Earth. This pressure is mainly 100 000 Newton which can crush our body but our bodies have the exact amount of pressure as the atmospheric pressure. On a mountain, as the ground rises above 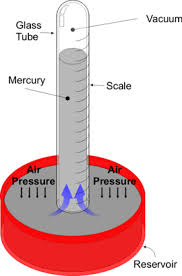 sea level, the atmospheric pressure decreases. The atmospheric pressure is expressed in the units; Atmosphere (atm), Pascals and Kilopascals (Pa and kPa), and in millimeters. The atmospheric pressure is measured by a barometer. This is sometimes called a torricilian in respect to its inventor Torreceli. The barometer is filled with mercury and is placed inverted on a trough filled with mercury. The cylinder is stood still by the atmospheric pressure. Measure the column of the mercury to calculate the atmospheric pressure in millimeters. The atmospheric pressure is not constant and can change.
sea level, the atmospheric pressure decreases. The atmospheric pressure is expressed in the units; Atmosphere (atm), Pascals and Kilopascals (Pa and kPa), and in millimeters. The atmospheric pressure is measured by a barometer. This is sometimes called a torricilian in respect to its inventor Torreceli. The barometer is filled with mercury and is placed inverted on a trough filled with mercury. The cylinder is stood still by the atmospheric pressure. Measure the column of the mercury to calculate the atmospheric pressure in millimeters. The atmospheric pressure is not constant and can change.
Pressure and Volume of gas
A gas’s pressure can be calculated if a manometer is conne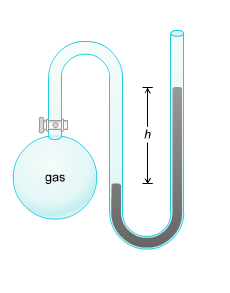 cted to a container filled with a gas. If the gas particles have low volume in the large container then it’s pressure may be equal to atmospheric pressure. This causes the liquid in the legs of the manometer to not loosen its height. If the gas is present in large volume, it will exert more pressure than the atmospheric air and push the liquid down the leg causing a decrease in the height of the column of liquid in one leg of the manometer but increases the height of the column of liquid in the other leg.
cted to a container filled with a gas. If the gas particles have low volume in the large container then it’s pressure may be equal to atmospheric pressure. This causes the liquid in the legs of the manometer to not loosen its height. If the gas is present in large volume, it will exert more pressure than the atmospheric air and push the liquid down the leg causing a decrease in the height of the column of liquid in one leg of the manometer but increases the height of the column of liquid in the other leg.
The same happens when the original amount of gas particles are present in a very small container.
You can calculate the relationship of volume with pressure by the formula:
(pressure x volume) first conditions = (pressure x volume) second condition
This is also known as Boyle’s Law ans is written as p1v1 = p2v2
With this I conclude this article. Thank You for reading and take care.
