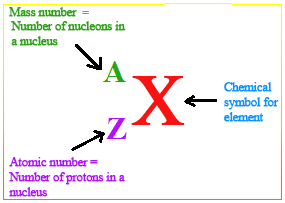
Subatomic particles
Atoms were considered to be the smallest particle a matter is composed of but this is not true. Atoms are composed of three subatomic particles; protons, neutrons and electrons. Electrons are 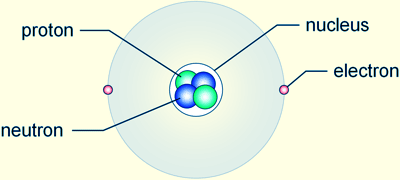 negatively charged subatomic particles which are found in the shells of the atom. The neutrons and protons are found in the nucleus of the atoms. Protons have a positive charge while neutrons carry no charge. Since atoms are not charged particles, that means that the number of protons and electrons are same.
negatively charged subatomic particles which are found in the shells of the atom. The neutrons and protons are found in the nucleus of the atoms. Protons have a positive charge while neutrons carry no charge. Since atoms are not charged particles, that means that the number of protons and electrons are same.
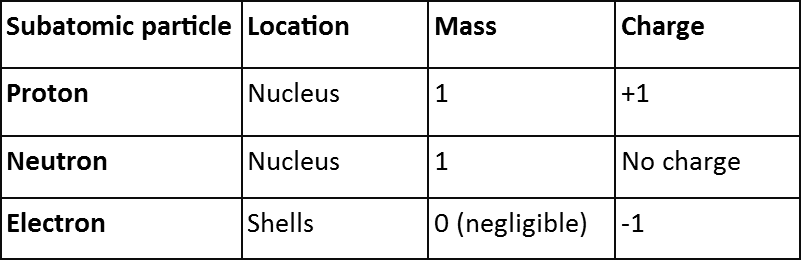 The mass of protons and neutrons are greater than electrons. If the mass of proton is 1 then the mass of electron is taken 0. Even though these two subatomic particles are different in masses they carry a same charge.
The mass of protons and neutrons are greater than electrons. If the mass of proton is 1 then the mass of electron is taken 0. Even though these two subatomic particles are different in masses they carry a same charge.
Neutrons make up the mass of an atom along with protons (not electrons since they are outside the nucleus). The only exception is hydrogen in which no neutron is found.
Proton number and Nucleon number
The number of protons found in a nucleus of the atom of an element, then that number is called its proton number/atomic number. Its symbol is Z.
The total mass of an atom build up by neutrons and protons make up the Nucleon number/Mass Number of the element. It is given the symbol A.
The neutron number is the total number of neutrons in an atom of the element and is given the symbol N.
The atomic number and mass number can be written as a subscript by the side of the symbol of an element.
Ions are formed when
the atoms gain or lose electrons. Losing an electron causes the positive charge of the proton to overpower the negative charge causing the formation of cation while gaining electron forms an anion which is negatively charged due to the same principle.
Electronic Configuration
The electrons are found in the shells of the atom. There are 4 shells in total. The equation given to calculate the amount of electrons each shell can take is; 2n^2. “n” means the number of shells. The 1st shell only holds 2 electrons, which forms a  duplet. The 2nd shell holds 8 electrons forming an octect. The 3rd can hold 18 and the fourth can hold up to 32. If these limits are crossed then the electrons are to be placed in the next shell. However calcium and potassium are free of such pattern because as their 3rd shells exceed 8 electrons it is placed in another shell.
duplet. The 2nd shell holds 8 electrons forming an octect. The 3rd can hold 18 and the fourth can hold up to 32. If these limits are crossed then the electrons are to be placed in the next shell. However calcium and potassium are free of such pattern because as their 3rd shells exceed 8 electrons it is placed in another shell.
The electronic configuration, is basically telling the amount of electrons in each shell in an element. This is given in a table by stating the number of electrons present with “,” in between without any space.
Isotopes
An element might have a constant number of protons and electrons but this is not true for neutrons. These neutrons make different masses of the same element causing the element to divide into smaller groups of the same element called isotopes. One example of isotope is chlorine. It has two masses. One type is 35 which is more common, exactly 75.77%. While the other is 37 which is 25% found. This gives rise to the two isotopes; chlorine-35 and chlorine-37. This is exactly the way every element is written to express its isotope, such as, carbon-12, carbon-13 etc.
Carbon-12 Scale
To ensure the consistency of an isotope, the isotope is compared to an ideal isotope which is normally carbon-12. This isotope is chosen because it is found in all the organic chemicals but not all the isotopes can be compared to this mass scale. So to ensure accuracy the isotopic mass of any element is compared to 1/12th that of the mass of carbon 12.
Relative atomic mass
The relative atomic mass shows the abundance of a mass and the mass of an atom. This is showed in the following equation:
mass1 x abundance/100 + mass2 x abundance/100
For chlorine we will use this equation. Using this equation tells us that the relative atomic mass of chlorine is 35.5. The equation could be altered just to cope with more isotopes of the same element.
Relative molecular mass
The relative molecular mass of an atom is the sum of the relative atomic masses of the atoms that build up the molecule. Atoms combine to form larger molecules of an element. To find the relative molecular mass we can just add the relative atomic masses of the atoms that make up the molecule:
Cl2 = 35.5 x 35.5 = 71
The above equation is the example of finding out the relative molecular mass of the chlorine molecule.
Relative Formula Mass
Ionic compounds are made of multiple elements so we use relative formula mass to find the mass of such compounds. Such as if you find the relative formula mass of NaCl (Sodium Chloride), you will find that it is 58.5 as Na (sodium)= 23 and Cl (chlorine) = 35.5 and 35.5 + 23 = 58.5.
Radioactive isotopes
Many isotopes are stable and will remain in the environment for several years. But there are some unstable or radioactive isotopes which break down into other elements. One example is carbon-14 which converts into nitrogen-14.