Thermal Energy is part of the Cambridge O-level syllabus. It is a pretty easy and basic chapter telling us about the ways in which Heat energy a.k.a thermal energy is transferred, which is mainly through conduction, convection and radiation.
Heat
Heat is mostly termed as energy. It is commonly said as thermal energy. Heat is transferred one way or another. This is because the heat energy tends to raise or  lower the temperature of an object until the temperature of the substance is equal to that of its environment.
lower the temperature of an object until the temperature of the substance is equal to that of its environment.
For example, if you place a cup of hot tea in a room and keep it there without any change in the environment, the tea will soon loose its heat and become cold. Similarly, placing an ice cold drink in the room will result in the drink becoming warmer than before. This is because heat energy is transferred from a hot region to a colder region. Other than that, heat keeps on transferring until the temperature of the substance is equal to that of the room temperature. Heat is transferred by conduction, convection and radiation.
Conduction
According to the kinetic particle theory, heat energy is converted into kinetic energy by the particles of the three states of matter. The particles in solid do not move about their position as they are packed together in a matrix but these particles vibrate and jostle other particles until every particle has received the kinetic energy and changed the state of matter. This process is conduction.
Almost every object can conduct heat. Some are poor conductors of heat which are called thermal insulators. The objects that are better in conducting heat are called thermal conductors. Almost all metals are conductors.
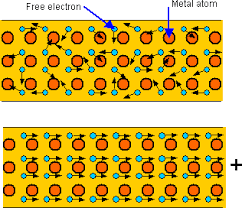 Metals tend to be the best at conducting heat and electricity. This is because of their structure. A metal has ions in its long matrix of atoms and ions. There are some electrons in metals that are called delocalized or free because they are not held by an ion. These electrons move around the metal structure freely. When metals are heated, the particles do vibrate but the free electrons move around the matrix of ions and atoms, these electrons spread the heat energy across the metal causing the metal to heat up faster than non metals.
Metals tend to be the best at conducting heat and electricity. This is because of their structure. A metal has ions in its long matrix of atoms and ions. There are some electrons in metals that are called delocalized or free because they are not held by an ion. These electrons move around the metal structure freely. When metals are heated, the particles do vibrate but the free electrons move around the matrix of ions and atoms, these electrons spread the heat energy across the metal causing the metal to heat up faster than non metals.
Convection
Convection is the process of heat transfer in liquids and gases. This is because the particles in liquid and gas have huge distances between each particle and are more free to move. In liquids and in gases convection currents are the main ways of transfer of heat energy.
In liquids, we can fill a beaker with water and place a solid dye in its bottom  corner in the liquid. Heat up the beaker over the Bunsen burner and notice as the dye diffuses into the liquid. The dye moves up and slowly moves down again. This is because the bottom the beaker is heated, the liquid in that region gains heat energy and loses its density, therefore it travels to the upper region, where the temperature is still low. The water from the top moves down to the hot region and gains heat energy. This movement allows heat to be transferred to the whole liquid until the liquid starts to boil and change its state to gas.
corner in the liquid. Heat up the beaker over the Bunsen burner and notice as the dye diffuses into the liquid. The dye moves up and slowly moves down again. This is because the bottom the beaker is heated, the liquid in that region gains heat energy and loses its density, therefore it travels to the upper region, where the temperature is still low. The water from the top moves down to the hot region and gains heat energy. This movement allows heat to be transferred to the whole liquid until the liquid starts to boil and change its state to gas.
In gas, the same happens as in the liquids. The best example is found by the land and sea breezes. During the day the sea heats up slowly and the land heats up quickly. The air above land, once heated, rises into the atmosphere. This rise creates a low pressure in the region causing the colder sea breezes to come and take its place until this also heats up and follows the cycle again and again. This  forms the sea breeze
forms the sea breeze
During the night time, the sea is slowly losing its heat while the land loses its heat much more quickly. This causes the air above land to cool down and create a high pressure region. The air above sea is hot and by the same principle, it will move towards the land and the cool air moves to the sea causing a land breeze.
Radiation
In space, the Sun and Earth are distance apart from each other and there is  vacuum between the Earth and the Sun but heat is still transferred to the Earth. This is because heat is transferred in the form of infra-red rays along with other rays emitted by the Sun. These infra red rays have short wavelength and is absorbed by the Earth. The soil absorbs the rays and emits them back to the atmosphere. This time the rays have a longer wavelength. These rays are absorbed by the greenhouse gases. The rays are again send back to Earth causing the Earth to heat up. This maintains the temperature of the Earth.
vacuum between the Earth and the Sun but heat is still transferred to the Earth. This is because heat is transferred in the form of infra-red rays along with other rays emitted by the Sun. These infra red rays have short wavelength and is absorbed by the Earth. The soil absorbs the rays and emits them back to the atmosphere. This time the rays have a longer wavelength. These rays are absorbed by the greenhouse gases. The rays are again send back to Earth causing the Earth to heat up. This maintains the temperature of the Earth.
This shows that heat is better radiated and absorbed by dark, and rough surfaces compared to shiny and smooth surfaces.
