Latent Heat and Specific Latent Heat
Some solids turn into liquids when they are heated. This is because the rise in heat energy causes rise in internal energy of the particles of the solid which then vibrate to break the inter-molecular bonds between each particle. As the solid is heated its temperature rises, but when the solid starts to melt to convert into liquid, at that point the temperature remains constant. This is because the heat energy is used to break the inter molecular forces which attract the particles towards each other. This is the point at which the temperature neither falls nor rises. This sequence is known as Latent Heat of fusion, Lf. The same is for liquids. When a liquid starts to boil, its temperature rises and evaporation is at its highest. But when the liquid is on the brink to becoming a gas, its temperature remains constant as heat energy is again used to break the forces of attraction between the particles. After this sequence the liquid turns into gas. This sequence is called as latent heat of vaporization, Lv.
The true definition of latent heat will be according to its type:
The amount of energy needed to convert a solid to liquid and vice versa without any change in temperature is called latent heat of fusion.
and;
The amount of energy needed to convert a liquid into gas and vice versa without any change in temperature is called latent heat of vaporization.
To calculate the latent heat of fusion of an object we multiply the specific latent heat of fusion with the mass of the object; Lf = m x lf.
Lf is latent heat of fusion.
m is mass of the object.
lf is specific latent heat of fusion ( it is written in lower case)
The same equation goes for the latent heat of vaporization; Lv = m x lv.
The specific latent heat of fusion is the amount of energy needed to convert 1 kg mass of an object from solid to liquid without any change in temperature.
The specific latent heat of vaporization is the amount of energy needed to convert 1 kg mass of liquid into gas without any change in temperature.
Just remember that the symbol to express Power is Watt and 1 Watt is equal to 1 Joules. The Joules symbol is used to express the latent heat.
Finding Specific Latent Heat
The specific latent heat of fusion of a pure substance is found by providing  energy to a pure solid and calculating the mass of the liquid formed. For example, a known mass of pure ice is placed in a funnel with a beaker under the funnel. When water starts to drop out of the funnel, the temperature of the ice can be taken 0°C. Then an electric heater is placed inside the crushed ice and a known amount of energy is provided. Since the heater is provided with electrical energy, it can be calculated efficiently. When the ice is completely converted into water then the mass of water is calculated using an electronic balance.
energy to a pure solid and calculating the mass of the liquid formed. For example, a known mass of pure ice is placed in a funnel with a beaker under the funnel. When water starts to drop out of the funnel, the temperature of the ice can be taken 0°C. Then an electric heater is placed inside the crushed ice and a known amount of energy is provided. Since the heater is provided with electrical energy, it can be calculated efficiently. When the ice is completely converted into water then the mass of water is calculated using an electronic balance.
To find the Specific latent heat of vaporization we heat up a liquid with an electric  heater and then find the mass lost from the initial mass of the liquid. For example; A beaker is placed on an electronic balance, it is filled with pure water and its mass is calculated excluding the tare value. An electric heater is placed and the substance is heated. Then we measure the loss of mass of the liquid over the amount of power used to heat the substance. Note that the loss of mass of the liquid is a gain in mass of gas, such as loss of 3g of liquid is an increase in 3 gram of gas.
heater and then find the mass lost from the initial mass of the liquid. For example; A beaker is placed on an electronic balance, it is filled with pure water and its mass is calculated excluding the tare value. An electric heater is placed and the substance is heated. Then we measure the loss of mass of the liquid over the amount of power used to heat the substance. Note that the loss of mass of the liquid is a gain in mass of gas, such as loss of 3g of liquid is an increase in 3 gram of gas.
Heat capacity and Specific Heat capacity
If you heat one iron block and one copper block, both having a mass of 5 grams then you will notice that one block heats up faster than the other. If you heat 2 blocks of copper, one having the mass of 5 grams while the other having 10 grams of mass, then again the same effect occurs. This shows that the heat capacity of an object depends upon the mass of an object and the material it is made of.
Heat capacity of an object is the amount of energy needed to raise the temperature of an object by 1 Kelvin or 1°C.
To calculate the Heat Capacity we use the equation; C = E/ΔQ
C is heat capacity.
E is total energy used.
ΔQ is change in temperature (initial temperature – end temperature). For Kelvin scale we use the symbol ΔΤ.
The specific heat capacity of an object is the amount of energy needed to raise the temperature of 1 kg of object by 1 K or 1°C. To find the Specific Heat Capacity of an object we use the equation; c = C/m or c = 1/m (E/ΔQ).
c is specific heat capacity which is always written in lower case.
m is mass of the object.
C is heat capacity.
Finding heat capacity
The heat capacity of a solid is found by placing an electric heater in the solid along with a thermometer. The initial temperature is calculated. The solid is then provided with an insulation to avoid any and loss of energy. The electric heater then provides the solid with a specific amount of energy. The rise in temperature is then calculated to find the heat capacity of the solid.
with a thermometer. The initial temperature is calculated. The solid is then provided with an insulation to avoid any and loss of energy. The electric heater then provides the solid with a specific amount of energy. The rise in temperature is then calculated to find the heat capacity of the solid.
For finding the heat capacity of a liquid, the liquid is poured in a container and is capped with a copper calorimeter. The initial temperature is measured by a thermometer. Then the liquid is heated with an electric heater and then the rise in temperature is measured. The problem with this is that the copper calorimeter absorbs some of the energy causing a little problem with measuring heat capacity.
finding the heat capacity of a liquid, the liquid is poured in a container and is capped with a copper calorimeter. The initial temperature is measured by a thermometer. Then the liquid is heated with an electric heater and then the rise in temperature is measured. The problem with this is that the copper calorimeter absorbs some of the energy causing a little problem with measuring heat capacity.
Thermal Expansion and Charles’ Law
Every substance expands when heated and contracts when cooled. In solids,  metals are best at this because these are good conductors of heat. The heat energy causes the metals to expand. This is why metal bridges tend to have rollers on one of its end to allow the bridge to expand a bit. A bimetal strip has two different types of metals, with one that expands sooner than the other causing the strip to bend.
metals are best at this because these are good conductors of heat. The heat energy causes the metals to expand. This is why metal bridges tend to have rollers on one of its end to allow the bridge to expand a bit. A bimetal strip has two different types of metals, with one that expands sooner than the other causing the strip to bend.
In liquids, these expand better than solids. In a flask filled with water and capped by a stopper with a hole in it, place your hand around it for several minutes and notice that a small amount of water comes out of it. This is because the hands heat up the water and it expands. Water has an unusual property. Once it cools down, it contracts slowly, but when reached to 4°C it expands again. This is thought to be due to the density of water. Due to this the water expands and allows marine life to live even if there is a sheet of ice on the surface of the water in colder environments.
Gases also expand with the rise of temperature and does so better than any solid or liquid. To test this, connect a flask filled with air to a U-tube with a dyed solution and then heat up the flask. Notice that the levels of the liquid change as the flask is heated.
When gases are cooled, the volume of the gases decrease gradually and when a gas is heated then the volume also increases. This is shown by the Charles’ Law.
And in accordance to the law we devise the equation; V ∝ T or V = constant x T
To find the constant = V/T
V is volume
T is total temperature


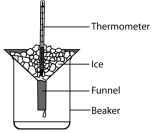 he melting point of a pure substance. A pure substance is essential for this because impurities in a substance alters the boiling point and the melting point of the substance. These impurities lowers the melting point and raise the boiling point.
he melting point of a pure substance. A pure substance is essential for this because impurities in a substance alters the boiling point and the melting point of the substance. These impurities lowers the melting point and raise the boiling point. the temperature and mark this as the melting point of the pure substance.
the temperature and mark this as the melting point of the pure substance. liquid in glass thermometer has a few properties which is very helpful for measuring temperature. The glass which makes the body of the capillary tube is thick acting as a magnifying glass for easy reading of the measurements. The bore was first made of glass but commonly is made of metal since metals are good conductors of heat. The
liquid in glass thermometer has a few properties which is very helpful for measuring temperature. The glass which makes the body of the capillary tube is thick acting as a magnifying glass for easy reading of the measurements. The bore was first made of glass but commonly is made of metal since metals are good conductors of heat. The  liquid inside the glass is either alcohol (ethanol) or mercury. Both have their disadvantages and advantages but mercury is more commonly used as it reacts to heat better than alcohol.
liquid inside the glass is either alcohol (ethanol) or mercury. Both have their disadvantages and advantages but mercury is more commonly used as it reacts to heat better than alcohol.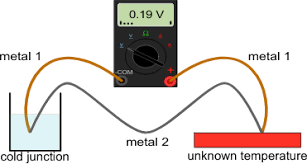 electrical device. The advantages for using this kind of thermometer is:
electrical device. The advantages for using this kind of thermometer is: Thermochromic or Thermocolor thermometer is more simple to use and is used for household purposes, mainly to check the temperature of an ill person. The thermometer has liquid crystals in it which change color due to rise in temperature.
Thermochromic or Thermocolor thermometer is more simple to use and is used for household purposes, mainly to check the temperature of an ill person. The thermometer has liquid crystals in it which change color due to rise in temperature.
 lower the temperature of an object until the temperature of the substance is equal to that of its environment.
lower the temperature of an object until the temperature of the substance is equal to that of its environment.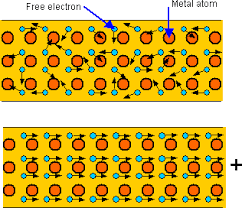 Metals tend to be the best at conducting heat and electricity. This is because of their structure. A metal has ions in its long matrix of atoms and ions. There are some electrons in metals that are called delocalized or free because they are not held by an ion. These electrons move around the metal structure freely. When metals are heated, the particles do vibrate but the free electrons move around the matrix of ions and atoms, these electrons spread the heat energy across the metal causing the metal to heat up faster than non metals.
Metals tend to be the best at conducting heat and electricity. This is because of their structure. A metal has ions in its long matrix of atoms and ions. There are some electrons in metals that are called delocalized or free because they are not held by an ion. These electrons move around the metal structure freely. When metals are heated, the particles do vibrate but the free electrons move around the matrix of ions and atoms, these electrons spread the heat energy across the metal causing the metal to heat up faster than non metals. corner in the liquid. Heat up the beaker over the Bunsen burner and notice as the dye diffuses into the liquid. The dye moves up and slowly moves down again. This is because the bottom the beaker is heated, the liquid in that region gains heat energy and loses its density, therefore it travels to the upper region, where the temperature is still low. The water from the top moves down to the hot region and gains heat energy. This movement allows heat to be transferred to the whole liquid until the liquid starts to boil and change its state to gas.
corner in the liquid. Heat up the beaker over the Bunsen burner and notice as the dye diffuses into the liquid. The dye moves up and slowly moves down again. This is because the bottom the beaker is heated, the liquid in that region gains heat energy and loses its density, therefore it travels to the upper region, where the temperature is still low. The water from the top moves down to the hot region and gains heat energy. This movement allows heat to be transferred to the whole liquid until the liquid starts to boil and change its state to gas. forms the sea breeze
forms the sea breeze vacuum between the Earth and the Sun but heat is still transferred to the Earth. This is because heat is transferred in the form of infra-red rays along with other rays emitted by the Sun. These infra red rays have short wavelength and is absorbed by the Earth. The soil absorbs the rays and emits them back to the atmosphere. This time the rays have a longer wavelength. These rays are absorbed by the greenhouse gases. The rays are again send back to Earth causing the Earth to heat up. This maintains the temperature of the Earth.
vacuum between the Earth and the Sun but heat is still transferred to the Earth. This is because heat is transferred in the form of infra-red rays along with other rays emitted by the Sun. These infra red rays have short wavelength and is absorbed by the Earth. The soil absorbs the rays and emits them back to the atmosphere. This time the rays have a longer wavelength. These rays are absorbed by the greenhouse gases. The rays are again send back to Earth causing the Earth to heat up. This maintains the temperature of the Earth.