Light is a form of energy which travels as an electromagnetic wave. We have already discussed the reflective properties of waves in the previous article about waves, light waves also reflect when they touch an opaque surface. We use light rays for this because these are easier to observe.
Reflection of Light
Lig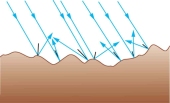 ht waves follow the same principle as any other wave. When a few parallel light waves strike a smooth, flat surface, it will change direction and move away from the surface, in parallel rays. This is the principle of reflection.
ht waves follow the same principle as any other wave. When a few parallel light waves strike a smooth, flat surface, it will change direction and move away from the surface, in parallel rays. This is the principle of reflection.
On a rough surface the light rays do reflect but they will be more scattered. The rays will not be parallel to each other.
If a light ray impacts on a plane mirror, which is opaque, then the light will reflect. An imaginary line is formed perpendicular to the surface of the mirror at the point where the ray touches the surface, this straight line is called the normal.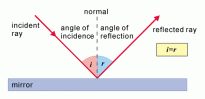
The ray moving towards the mirror is called the incident ray which is incident on the plane mirror and the ray moving away from the mirror is the reflected ray.
The angle formed between the incident ray and the normal is called the angle of incidence, i.
The angle formed between the reflected ray and the normal is called the angle of reflection, r.
The angle of incidence and the angle of reflection is always equal.
Images formed By reflection
When you place a candle in front of the mirror and then look towards the mirror you will be able to see an image of the candle.
An image can be either real or virtual. A virtual image cannot be projected on to a screen. If you place the same candle in front of the wall you will notice that no image is formed on the wall.
A real image can be projected on the a screen. If you place a projector in front of 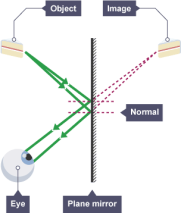 the wall, and add the tape, then turn on the projector, it will form an image on the wall.
the wall, and add the tape, then turn on the projector, it will form an image on the wall.
To you, it will appear as if the image is coming from behind the mirror although it is not true. In a diagram, we will show the rays as dotted lines that form the image behind the mirror.
In a diagram, you have to create two rays of light and at the point where the two rays converge an image is formed. Since the image is not really behind the mirror we will say that the image is virtual.
The virtual image will be of the same size as the object from which the light rays are coming from and it will be inverted (it will be back to front) and also will be at the same distance from the mirror as the object.
Refraction of light
All waves can reflect and refract. The reason waves refract is because of the sudden change of speed. Light waves travel fastest in vacuum, while it travels in about the same speed in air as in vacuum.
When it enters another transparent medium or substance that is more dense than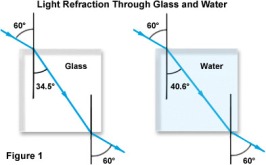 the medium light is originally travelling in, the light wave slows down and then changes its direction. This change in direction is called refraction.
the medium light is originally travelling in, the light wave slows down and then changes its direction. This change in direction is called refraction.
If you place a transparent glass block on a platform and point a laser light on to it at an angle the light will refract.
You can create a normal here where the light touches the glass block. Measure the angle of incidence. In case of refraction, the angle of refraction is used, which is in between the refracted ray and the normal.
A point to be noted is that when light enters a denser medium from a less dense medium, the angle of refraction will always be smaller than the angle of incidence.
When the light wave moves from more dense to a less dense medium, its angle of refraction will always be greater than the angle of incidence.
If the light ray is moving straight, perpendicular to the glass block surface, then it will not refract but pass through.
The refractive property depends upon the thickness or, more importantly, the refractive index, n, of the medium that light travels in.
The refractive index is the ratio of the sine of the angle of incidence to the sine of angle of refraction: n = sin i/sin r. This equation is called Snell’s Law which is named after the Dutch scientist Willebrord Snellius known mainly as Snell.
The refractive index of a medium determines the angle of refraction of the light ray. The higher the refractive index, the angle of refraction will always be smaller than the angle of incidence.
If the refractive index is low, then the angle of refraction will be larger than the angle of incidence.
Total internal reflection
When light enters a less dense medium it has a greater angle of refraction than angle of incidence. When the angle of incidence increases then so will the angle of refraction.
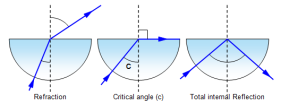 There comes a point when the angle of refraction becomes 90°. At this point the angle of incidence is called the critical angle.
There comes a point when the angle of refraction becomes 90°. At this point the angle of incidence is called the critical angle.
If the critical angle is further increased then the light will obey the laws of reflection. This is because when the light refracts, there is still a small amount of light that is reflected of the surface of the denser medium.
The light at this point is completely reflected which is why this situation is called the total internal reflection.
To find the critical angle we use the equation, c=1/n. c represents critical angle, 1 represents the sine of angle of refraction because angle of refraction is always 90 and sin(90) is always equal to 1, n represents the refractive index.
Lenses
Lenses can either be concave or convex. These lenses have different refractive properties.
The convex lens tend to cause parallel rays of light to refract and converge at a certain point. The concave lens causes parallel rays of light to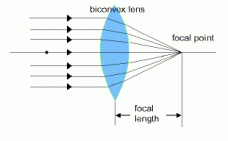 refract and diverge.
refract and diverge.
The point at which the lens converge, an image is formed, and this point is also known as the principle focus or focal point. The distance from between the focal point and the lens is called the focal length.
The image formed might be real or virtual depending upon the lens as well as the distance at which an object is placed. In a concave lens, the image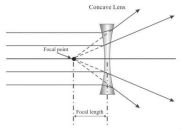 formed is always virtual, because the image appears to be coming from across the lens.
formed is always virtual, because the image appears to be coming from across the lens.
We will make dotted lines to see the light converge at a certain point, even though this convergence is imaginary, this will still become a focal point.
Ray diagrams
To understand this, we can also make a ray diagram. The y-axis of the ray diagram is the lens and the x-axis will be the principle axis.
If an object is placed in front of the lens, light rays will bounce off the object and enter the lens and then refract.
The point at which the light rays will pass through the principle axis, that point will be the principle focus or focal point.
The length from this point to the lens axis will be known as the focal length. The image will always be formed below the principle axis if the image is real.
To construct the light beams on the diagram, make a light ray from the object to 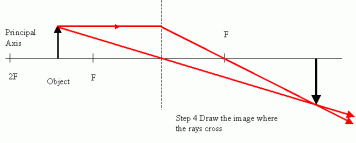 the lens axis parallel to the principle axis and then from the lens axis change its direction towards the principle axis.
the lens axis parallel to the principle axis and then from the lens axis change its direction towards the principle axis.
Make another ray of light straight through the optical center (the point at which the principle axis and the lens axis meet). This ray of light will not refract at all.
You can also make a third ray of light on the diagram straight through the principle axis, it should touch the lens axis and then it will refract and move parallel to the principle axis.
Real Image formed on ray diagrams
The point at which these rays of light converge, an image is formed. Mark the length of this point to the principle axis and this will be the magnification of the image.
To find the magnification of the image formed we will divide the length of the image to the length of the object. With this we can say that magnification is the ratio of the height of the object to the height of the image; m = hi/ho. m is for magnification, hi is for height of image, ho is the height of object.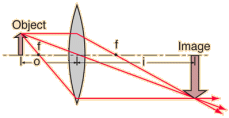
We can also find magnification by dividing the distance of the image from the lens to the distance of the object to the lens; m=v/u. v is for distance of image while u is for distance of object.
The height of the image formed will depend upon the distance of the object from the lens. If the object 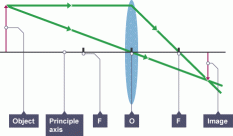 is at the focal point, the image formed will be of the same size as the object.
is at the focal point, the image formed will be of the same size as the object.
If the object moves away from the focal point the image will magnify. If the object moves away twice the focal length, the image will diminish.
Virtual Images formed from Lens
In a ray diagram, if you move the object closer to the convex lens, then the rays of light will refract but will not converge, to an observer the image formed will be at the back of the lens.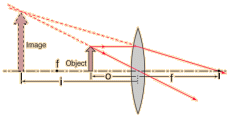
On the diagram we would draw dotted lines towards the back of the object to a point where the light rays will converge, here an image is formed, since this is the point where the light is seemingly coming from (it’s not actually) this will make a virtual image which is magnified.
 A concave lens causes the parallel beam of light to diverge. Because the parallel beam of light diverges, straight dotted lines (to represent the imaginary rays of light) are drawn from the diverged rays to converge them on the left of the ray diagram above the principle axis.
A concave lens causes the parallel beam of light to diverge. Because the parallel beam of light diverges, straight dotted lines (to represent the imaginary rays of light) are drawn from the diverged rays to converge them on the left of the ray diagram above the principle axis.
The point where the light converges, that point is where the virtual image is formed.
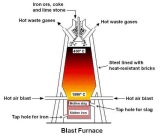 temperature. The hot air goes out through the pipes on the top of the furnace leading out. The upper hole of the furnace is where the products; coke, iron ore and limestone are added. The gases heat up the top of the furnace to 900°C and the lower part is heated to 2400°C. The carbon content in the coke reacts with the hot air in the furnace to produce carbon dioxide and carbon monoxide. The limestone is useful for helping protect
temperature. The hot air goes out through the pipes on the top of the furnace leading out. The upper hole of the furnace is where the products; coke, iron ore and limestone are added. The gases heat up the top of the furnace to 900°C and the lower part is heated to 2400°C. The carbon content in the coke reacts with the hot air in the furnace to produce carbon dioxide and carbon monoxide. The limestone is useful for helping protect
 reduce its value. The corrosion of metals are called rust. It can be prevented by covering the metal with paints, plastic or even metals which are above the metallic material used in the reactivity series. The galvanization is the process in which iron is covered with zinc to act as protection. If the layer is damaged and water or air pass through, the zinc acts as the anode and the iron as the cathode which keeps the iron protected even after damage. Tinning is the same process, only difference is that iron is protected by tin which is lower than iron in reactivity series. This makes the iron the anode and tin the cathode once damaged making the rusting process faster.
reduce its value. The corrosion of metals are called rust. It can be prevented by covering the metal with paints, plastic or even metals which are above the metallic material used in the reactivity series. The galvanization is the process in which iron is covered with zinc to act as protection. If the layer is damaged and water or air pass through, the zinc acts as the anode and the iron as the cathode which keeps the iron protected even after damage. Tinning is the same process, only difference is that iron is protected by tin which is lower than iron in reactivity series. This makes the iron the anode and tin the cathode once damaged making the rusting process faster.
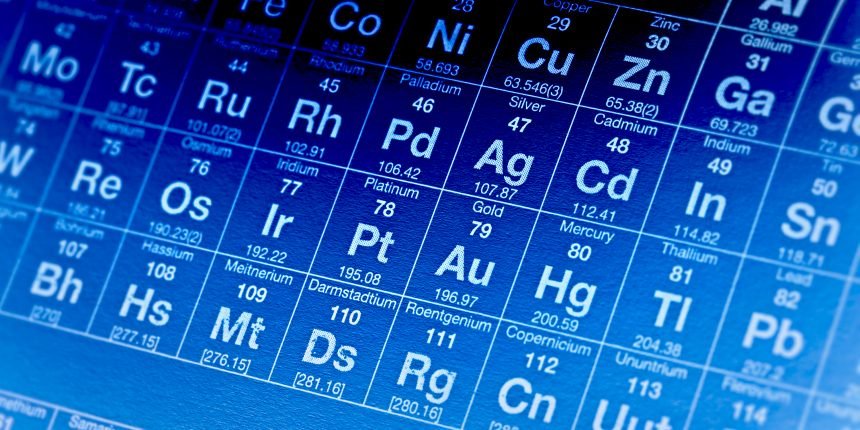
 have their atomic symbol to be abbreviated to their first alphabet in upper case letters but many have two alphabets such as Cu, C, Ca, these all have the alphabet C but represent different elements. The second alphabet is there to distinguish the elements from each other to avoid any confusion. The periods in the periodic table represent the number of shells there are in the element present in the period. The groups in the Periodic table represent the number of valence electron found on the outermost shell of the elements present.
have their atomic symbol to be abbreviated to their first alphabet in upper case letters but many have two alphabets such as Cu, C, Ca, these all have the alphabet C but represent different elements. The second alphabet is there to distinguish the elements from each other to avoid any confusion. The periods in the periodic table represent the number of shells there are in the element present in the period. The groups in the Periodic table represent the number of valence electron found on the outermost shell of the elements present.
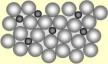 initial reactants. The reason for such a difference in physical properties is that the all elements have atoms which are of different sizes which is why when two metals or non metals combine the particles are forced into shape, but they cannot be gain the same properties as before such as an alloy of copper and zinc cannot be malleable and ductile. This is because the zinc atom is large in size than copper atom.
initial reactants. The reason for such a difference in physical properties is that the all elements have atoms which are of different sizes which is why when two metals or non metals combine the particles are forced into shape, but they cannot be gain the same properties as before such as an alloy of copper and zinc cannot be malleable and ductile. This is because the zinc atom is large in size than copper atom.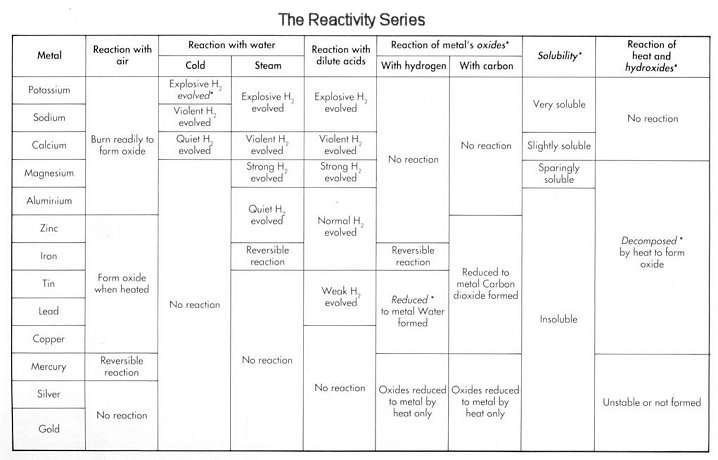 The reactivity series is a group of metals arranged according to their reactivity towards various materials with potassium being the most reactive and gold being the least reactive. This series also have hydrogen and carbon in it. The Group I and II are most reactive and then aluminium and then the transition elements.
The reactivity series is a group of metals arranged according to their reactivity towards various materials with potassium being the most reactive and gold being the least reactive. This series also have hydrogen and carbon in it. The Group I and II are most reactive and then aluminium and then the transition elements.
 This reaction is reversible which is we must consider the position of the dynamic equilibrium and the factors which affect the position. The forward reaction is exothermic so the temperature should be low and the pressure must be high to produce highest yield. This is a problem since the machines used to raise pressure are expensive and the low temperature slows down the rate of reaction. The rate of reaction is increased by adding an iron catalysts. The catalysts lower the need of activation energy and thus fasten the process. To further maximise its output, the Haber process is used. In this process the reactants flow to a reactor where they are combined together and then flow to a condenser where the ammonia formed is condensed out while the unreacted nitrogen and hydrogen flow back into the reactor which results in a higher yield.
This reaction is reversible which is we must consider the position of the dynamic equilibrium and the factors which affect the position. The forward reaction is exothermic so the temperature should be low and the pressure must be high to produce highest yield. This is a problem since the machines used to raise pressure are expensive and the low temperature slows down the rate of reaction. The rate of reaction is increased by adding an iron catalysts. The catalysts lower the need of activation energy and thus fasten the process. To further maximise its output, the Haber process is used. In this process the reactants flow to a reactor where they are combined together and then flow to a condenser where the ammonia formed is condensed out while the unreacted nitrogen and hydrogen flow back into the reactor which results in a higher yield. Sulphur is used to make sulphuric acid which is an important product. It is made by the Contact process. In this process the sulphur is first mixed with air to react with oxygen to form sulphur dioxide. The sulphur dioxide is then mixed with more oxygen to form sulphur trioxide. This reaction is reversible and
Sulphur is used to make sulphuric acid which is an important product. It is made by the Contact process. In this process the sulphur is first mixed with air to react with oxygen to form sulphur dioxide. The sulphur dioxide is then mixed with more oxygen to form sulphur trioxide. This reaction is reversible and  exothermic which is why there is a need to raise the pressure (just a little in this case) and to lower the temperature. A reactor is used which has almost 4 bases coated with the catalyst vanadium oxide. The sulphur dioxide gas is pumped through the reactor which cools down by the first 2 bases and then is oxidised by the 3rd and 4rth base. The sulphur trioxide is then mixed with sulphuric acid to form an oleum which is then mixed with a few amounts of water to form a more concentrated sulphuric acid solution. To form a dilute solution a few amounts of oleum is mixed with a large amount of water along with stirring. We use small quantities of either materials because the reaction between sulphuric acid and water is so exothermic that the water and sulphur dioxide bubbles up and spread water out of the containers making the process dangerous.
exothermic which is why there is a need to raise the pressure (just a little in this case) and to lower the temperature. A reactor is used which has almost 4 bases coated with the catalyst vanadium oxide. The sulphur dioxide gas is pumped through the reactor which cools down by the first 2 bases and then is oxidised by the 3rd and 4rth base. The sulphur trioxide is then mixed with sulphuric acid to form an oleum which is then mixed with a few amounts of water to form a more concentrated sulphuric acid solution. To form a dilute solution a few amounts of oleum is mixed with a large amount of water along with stirring. We use small quantities of either materials because the reaction between sulphuric acid and water is so exothermic that the water and sulphur dioxide bubbles up and spread water out of the containers making the process dangerous.
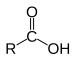 s as the rest of the homologous series. The carboxylic acids use the suffix ‘anoic’ and along with the word ‘acid’. Carboxylic acids are generally weak acids becuase these have chains of carbon in them, when the chains go long and long they become more insoluble in water. Even the soluble acids are weak acids because the range of dissociation of the acids into ions is so small and the reactions a re
s as the rest of the homologous series. The carboxylic acids use the suffix ‘anoic’ and along with the word ‘acid’. Carboxylic acids are generally weak acids becuase these have chains of carbon in them, when the chains go long and long they become more insoluble in water. Even the soluble acids are weak acids because the range of dissociation of the acids into ions is so small and the reactions a re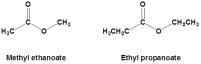 versible. Carboxylic acids react with alcohol to form esters. Esters are formed when these two homologous series combine and the new compound is named after the two. The name of the alcohol is used first and the name of the carboxylic acid is used after, for example ‘ethyl ethanoate’.
versible. Carboxylic acids react with alcohol to form esters. Esters are formed when these two homologous series combine and the new compound is named after the two. The name of the alcohol is used first and the name of the carboxylic acid is used after, for example ‘ethyl ethanoate’.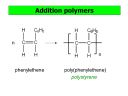 lymers with two different monomers are called copolymers. The polymers have two ways of formation. One is the condensation method. In this method when monomers join together there is molecule which is released from the reaction which is commonly but not always water. The other method is the addition method, in which the alkenes break their carbon-carbon double bond to form longer chains of carbon. The products are named poly(name of the alkene). There are two types of polymers commonly used across the globe. The poly
lymers with two different monomers are called copolymers. The polymers have two ways of formation. One is the condensation method. In this method when monomers join together there is molecule which is released from the reaction which is commonly but not always water. The other method is the addition method, in which the alkenes break their carbon-carbon double bond to form longer chains of carbon. The products are named poly(name of the alkene). There are two types of polymers commonly used across the globe. The poly amide which is more comm
amide which is more comm only known as nylon. The nylon has long chains of carbon which make it hard and so it is combined with cotton to produce a better clothe. The second is the polyester which is also a famous fabric material used.
only known as nylon. The nylon has long chains of carbon which make it hard and so it is combined with cotton to produce a better clothe. The second is the polyester which is also a famous fabric material used.
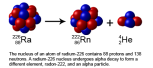 the radioactive isotopes, have a radioactive nuclide which slowly loses mass to form gamma radiation. Many other types of radiation are emitted by various materials. In case of an alpha particle emitter, the isotope mass loses its
the radioactive isotopes, have a radioactive nuclide which slowly loses mass to form gamma radiation. Many other types of radiation are emitted by various materials. In case of an alpha particle emitter, the isotope mass loses its 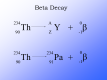 protons and neutrons to form a helium nucleus. The orginal element would lose 4 nucleon number and 2 proton numbers.
protons and neutrons to form a helium nucleus. The orginal element would lose 4 nucleon number and 2 proton numbers.
 along with other scientists, to give a description of the atoms. He suggested that atoms were small invisible and indivisible atoms which made up to be elements, atoms are neither created nor destroyed, atoms of same elements are identical and atoms of different elements are different in mass and size and that atoms combine to form compounds in small whole numbers. This was not entirely correct. He gave a supposed structure of the atom to be like a ball.
along with other scientists, to give a description of the atoms. He suggested that atoms were small invisible and indivisible atoms which made up to be elements, atoms are neither created nor destroyed, atoms of same elements are identical and atoms of different elements are different in mass and size and that atoms combine to form compounds in small whole numbers. This was not entirely correct. He gave a supposed structure of the atom to be like a ball. used a discharge tube which had a cathode and an anode, with two opposite charged electric plates. The discharge tube had inert gas in it which was at low pressure. The discharge tube had a high voltage of about 1500 V. He observed that the cathode rays travelled directly towards the anode and through a small hole travelled further on. It got repelled away by the negatively charged plate and attracted towards the positively charged plate. The
used a discharge tube which had a cathode and an anode, with two opposite charged electric plates. The discharge tube had inert gas in it which was at low pressure. The discharge tube had a high voltage of about 1500 V. He observed that the cathode rays travelled directly towards the anode and through a small hole travelled further on. It got repelled away by the negatively charged plate and attracted towards the positively charged plate. The 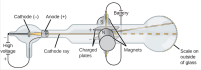 cathode rays usually end up travelling straight without the plates. He had discovered the electrons. He then test with other gases at low pressures as well and gave following conclusions after a few more experiments; The cathode rays must be negatively charged as it got repelled by the negatively charged plate but attracted towards the positively charged plate.The cathode rays must carry a charge as they got deflected by both the magnetic and electric field. The particles present in the cathode rays must be present in all atoms. Thompson then provided another model of the atom, saying that atoms had electrons in them but since they are neutral the rest of the mass must be positive. He also identified the mass of the electrons to be the same as always.
cathode rays usually end up travelling straight without the plates. He had discovered the electrons. He then test with other gases at low pressures as well and gave following conclusions after a few more experiments; The cathode rays must be negatively charged as it got repelled by the negatively charged plate but attracted towards the positively charged plate.The cathode rays must carry a charge as they got deflected by both the magnetic and electric field. The particles present in the cathode rays must be present in all atoms. Thompson then provided another model of the atom, saying that atoms had electrons in them but since they are neutral the rest of the mass must be positive. He also identified the mass of the electrons to be the same as always.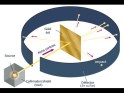 thin gold sheet and an alpha particle emitter. He bombarded the gold sheet with alpha particles and observed parts of the fluorescent sheet glow. The glowing points indicated the path taken by the alpha particles. He noticed that a lot of the alpha particles went straight through the gold sheet to reach the fluorescent sheet directly behind the gold sheet but some were at an angle away from the straight path and some were completely repelled backwards. He deduced that the alpha particles had passed through empty space which made up the nucleus of the
thin gold sheet and an alpha particle emitter. He bombarded the gold sheet with alpha particles and observed parts of the fluorescent sheet glow. The glowing points indicated the path taken by the alpha particles. He noticed that a lot of the alpha particles went straight through the gold sheet to reach the fluorescent sheet directly behind the gold sheet but some were at an angle away from the straight path and some were completely repelled backwards. He deduced that the alpha particles had passed through empty space which made up the nucleus of the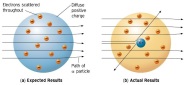 atom and that there must be a subatomic particle with a positive charge which repelled the alpha particles. Those which were deflected backwards must have come in direct contact with the proton. He then proposed a new model for the atomic structure.
atom and that there must be a subatomic particle with a positive charge which repelled the alpha particles. Those which were deflected backwards must have come in direct contact with the proton. He then proposed a new model for the atomic structure.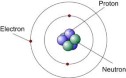 beryllium and saw that it emitted rays like the gamma rays which were extremely penetrating but were not deflected in the magnetic and the electric field making this clear that these rays were neutral. He had discovered neutron and made many conclusions with this.
beryllium and saw that it emitted rays like the gamma rays which were extremely penetrating but were not deflected in the magnetic and the electric field making this clear that these rays were neutral. He had discovered neutron and made many conclusions with this.
 the cells and causing mutation which may cause a tumor or trigger cancer.
the cells and causing mutation which may cause a tumor or trigger cancer.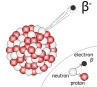
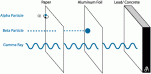 Gamma radiation are waves which are part of an electromagnetic spectrum. These waves have a high frequency and very low wavelength. These waves are neither positive nor negative. Several centimeters of dense metal is required. It is very penetrating to the human body which is why it is most dangerous.
Gamma radiation are waves which are part of an electromagnetic spectrum. These waves have a high frequency and very low wavelength. These waves are neither positive nor negative. Several centimeters of dense metal is required. It is very penetrating to the human body which is why it is most dangerous.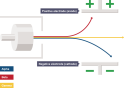 an electric field, the alpha radiation is deflected towards the negative field because it is attracted towards it but is deflected by the positive field. The beta particles are deflected by the negative field and attracted towards the positive field, since the beta particles are very small in mass compared to alpha particles they are pushed in greater amount than alpha particles. Gamma radiation is not deflected but passes straight through both the magnetic field and the electric field.
an electric field, the alpha radiation is deflected towards the negative field because it is attracted towards it but is deflected by the positive field. The beta particles are deflected by the negative field and attracted towards the positive field, since the beta particles are very small in mass compared to alpha particles they are pushed in greater amount than alpha particles. Gamma radiation is not deflected but passes straight through both the magnetic field and the electric field. it. The radiation reacts with the photographic film which is sealed from light. These photographic films help detect the amount of radiation the wearer is exposed to and is replaced regularly.
it. The radiation reacts with the photographic film which is sealed from light. These photographic films help detect the amount of radiation the wearer is exposed to and is replaced regularly. purposes, cloud chambers are used to detect the way the radiation particles move about. A cloud chamber has moist air which condenses on to the ionised air.
purposes, cloud chambers are used to detect the way the radiation particles move about. A cloud chamber has moist air which condenses on to the ionised air.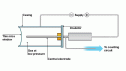 enters the casing, the radiation ionizes the air which then touches the wire passing on a current. An electronic pulse counter is connected with the tube to count the level of radiation.
enters the casing, the radiation ionizes the air which then touches the wire passing on a current. An electronic pulse counter is connected with the tube to count the level of radiation.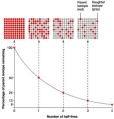 half-life. The formula for half-life is t1/2. If radioactive decay occurs, the isotope then emits radiation throughout. The fall of its activity can be obtained by measuring its activity at different times. The count rate falls as the element decays and so the amount of time it takes for the count rate to fall to a value half to the initial value is its half-life.
half-life. The formula for half-life is t1/2. If radioactive decay occurs, the isotope then emits radiation throughout. The fall of its activity can be obtained by measuring its activity at different times. The count rate falls as the element decays and so the amount of time it takes for the count rate to fall to a value half to the initial value is its half-life. the count rate measured. The radiation is most commonly the result of the gas radon being released from beneath the Earth. To measure the count rate to an accuracy, first measure the count rate of the background radiation and subtract it from the count rate of the intended object.
the count rate measured. The radiation is most commonly the result of the gas radon being released from beneath the Earth. To measure the count rate to an accuracy, first measure the count rate of the background radiation and subtract it from the count rate of the intended object.
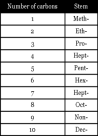 carbon. Two homologous series will be contained with in the context of this article; the Alkanes and the Alkenes. Each homologous series have their own functional group but they all use the same prefix to define the number of carbon atoms involved in each chemical, but the suffix is always different, the suffix will pronounce according to the homologous series the chemical finds itself in.
carbon. Two homologous series will be contained with in the context of this article; the Alkanes and the Alkenes. Each homologous series have their own functional group but they all use the same prefix to define the number of carbon atoms involved in each chemical, but the suffix is always different, the suffix will pronounce according to the homologous series the chemical finds itself in. different physical and chemical properties, such chemicals are called isomers of alkanes.
different physical and chemical properties, such chemicals are called isomers of alkanes.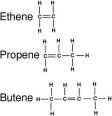

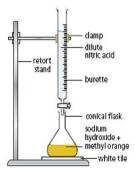 acids with bases. In any case the most affective way of carrying out the reactions is by the use of titration method. This method involves the apparatus; a conical flask and a burette. The burette is filled with an acid while the conical flask is placed underneath it with a base, there is an indicator mixed in it. The acid is poured to the point where the color of the mixture changes, the mixture is stirred along the reaction. When this happens the initial chemicals are again used according to the new measurements and then the mixture is heated and then set to cool and crystallize. This is done because the last mixture has an indicator in it which makes it useless because the salt is needed in pure form.
acids with bases. In any case the most affective way of carrying out the reactions is by the use of titration method. This method involves the apparatus; a conical flask and a burette. The burette is filled with an acid while the conical flask is placed underneath it with a base, there is an indicator mixed in it. The acid is poured to the point where the color of the mixture changes, the mixture is stirred along the reaction. When this happens the initial chemicals are again used according to the new measurements and then the mixture is heated and then set to cool and crystallize. This is done because the last mixture has an indicator in it which makes it useless because the salt is needed in pure form.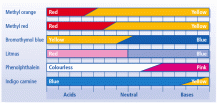 an acidic or alkaline solution. But such indicators are not useful if we were to detect the exact amount of acidity or basicity of the solution. For such reasons we use the universal indicator which has many indicators joined up to form one whole scale which can detect various ranges of acidity and basicity. The
an acidic or alkaline solution. But such indicators are not useful if we were to detect the exact amount of acidity or basicity of the solution. For such reasons we use the universal indicator which has many indicators joined up to form one whole scale which can detect various ranges of acidity and basicity. The
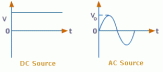 represent a direct current.
represent a direct current.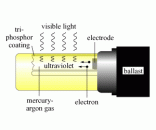 tungsten can react with oxygen and may cause an explosion.
tungsten can react with oxygen and may cause an explosion.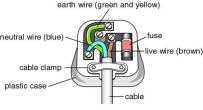
 container, these wires can only carry a certain amount of current like 5 A, or 7A but whatever the limit, if the current exceeds the limit, the wire melts causing the circuit to be broken. This is useful as in an appliance there are many components which make up to a lot of resistance but sometimes wires get cross connected causing a new circuit being made which reduces the resistance of the circuit and therefore increases the current. This is very dangerous and can give the user a shock if he/she touches the appliance. To avoid this fuses are plugged in which break up the circuit in case of an increase in current.
container, these wires can only carry a certain amount of current like 5 A, or 7A but whatever the limit, if the current exceeds the limit, the wire melts causing the circuit to be broken. This is useful as in an appliance there are many components which make up to a lot of resistance but sometimes wires get cross connected causing a new circuit being made which reduces the resistance of the circuit and therefore increases the current. This is very dangerous and can give the user a shock if he/she touches the appliance. To avoid this fuses are plugged in which break up the circuit in case of an increase in current.
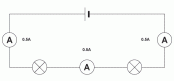 We can see the effect connecting components either way if we connect two bulbs as such. If connecting two bulbs in series, we can connect an ammeter on either point of the circuit to find that the current is same
We can see the effect connecting components either way if we connect two bulbs as such. If connecting two bulbs in series, we can connect an ammeter on either point of the circuit to find that the current is same 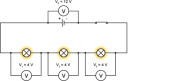 everywhere on the circuit. In case we connect a voltmeter in parallel to the bulbs, we can see that the p.d across each bulb is same and the total p.d of the bulbs make up the potential difference equal to the battery itself. By connecting more bulbs causes all the bulbs to lose their brightness soon and will break.
everywhere on the circuit. In case we connect a voltmeter in parallel to the bulbs, we can see that the p.d across each bulb is same and the total p.d of the bulbs make up the potential difference equal to the battery itself. By connecting more bulbs causes all the bulbs to lose their brightness soon and will break.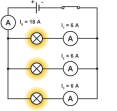 In a parallel circuit, the amount of components connected will not matter to much but if you keep on adding components like a bulb they will soon burn out. If one bulb burns out this does not affect the other bulbs like it does in a series circuit. This is because the parallel circuit makes up two circuits in principle, due to this the current gains two paths to move.
In a parallel circuit, the amount of components connected will not matter to much but if you keep on adding components like a bulb they will soon burn out. If one bulb burns out this does not affect the other bulbs like it does in a series circuit. This is because the parallel circuit makes up two circuits in principle, due to this the current gains two paths to move.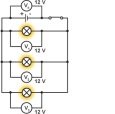 different, the sum of the two currents make up the total current. If a voltmeter is connected across the circuit, then the p.d across both components will be equal to the battery itself. This proves that the parallel circuits do not last as long as a series circuit but will be more efficient.
different, the sum of the two currents make up the total current. If a voltmeter is connected across the circuit, then the p.d across both components will be equal to the battery itself. This proves that the parallel circuits do not last as long as a series circuit but will be more efficient. A diode is made by silicon which is a semi-conductor. What a diode does is that it controls the direction of current flow. If a diode is in a forward position, this position is called forward biased, then the diode will help move the current forward only and no other direction. If it is connected in reverse then the diode will be negative biased and no current flows through the circuit.
A diode is made by silicon which is a semi-conductor. What a diode does is that it controls the direction of current flow. If a diode is in a forward position, this position is called forward biased, then the diode will help move the current forward only and no other direction. If it is connected in reverse then the diode will be negative biased and no current flows through the circuit. know whether a circuit is working or not. Today many led bulbs can be bought as an alternative to a conventional bulb but they are expensive but much more efficient to the conventional bulbs.
know whether a circuit is working or not. Today many led bulbs can be bought as an alternative to a conventional bulb but they are expensive but much more efficient to the conventional bulbs. A thermistor is resistor which depends upon the temperature. When the temperature increases then the resistance decreases and vice versa.
A thermistor is resistor which depends upon the temperature. When the temperature increases then the resistance decreases and vice versa.
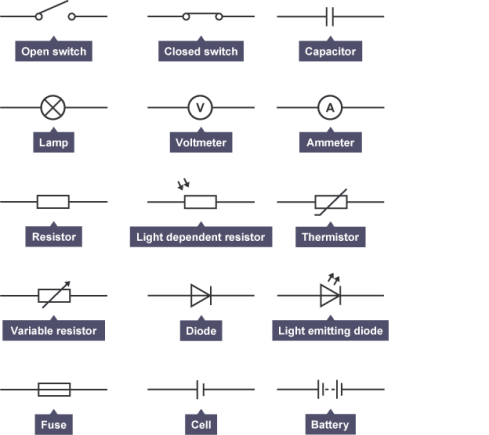
 ammeter is a device used to measure current passing through a circuit. There are two types of ammeter, one is analogue which has a needle and a scale on it but a digital ammeter is more commonly used due to its diverse use. The digital ammeter can be connected to a multimeter for other uses such as measuring Volts. A digital ammeter may have a range of 0 – 10A which can by modified by a shunt. A shunt has thin wire in it which can divert the current to flow through it allowing the
ammeter is a device used to measure current passing through a circuit. There are two types of ammeter, one is analogue which has a needle and a scale on it but a digital ammeter is more commonly used due to its diverse use. The digital ammeter can be connected to a multimeter for other uses such as measuring Volts. A digital ammeter may have a range of 0 – 10A which can by modified by a shunt. A shunt has thin wire in it which can divert the current to flow through it allowing the ammeter itself to measure a small amount of current, this is how the range of an ammeter increases. For example a shunt has been set at 90%, then the range of the ammeter will be increased to 0 – 1A. Ammeters are connected in a series circuit.
ammeter itself to measure a small amount of current, this is how the range of an ammeter increases. For example a shunt has been set at 90%, then the range of the ammeter will be increased to 0 – 1A. Ammeters are connected in a series circuit. y different currents flow through them. This is because each conductor has different resistance towards the flow of the electrons. Resistance is basically the ease at which electrons can flow through a conductor. The easier it is for the elctrons to flow the less resistance there is against it. Resistance is measured by ohms, Ω.
y different currents flow through them. This is because each conductor has different resistance towards the flow of the electrons. Resistance is basically the ease at which electrons can flow through a conductor. The easier it is for the elctrons to flow the less resistance there is against it. Resistance is measured by ohms, Ω. are two types of resistors fixed resistors and variable resistors. The fixed resistors have a fixed resistance but variable resistors can have a varied amount of resistance. A Light Dependant Resistor (LDR) depends upon light. If it is placed in front of a light source, its resistance drops while placing it in the dark will increase its resistance.
are two types of resistors fixed resistors and variable resistors. The fixed resistors have a fixed resistance but variable resistors can have a varied amount of resistance. A Light Dependant Resistor (LDR) depends upon light. If it is placed in front of a light source, its resistance drops while placing it in the dark will increase its resistance.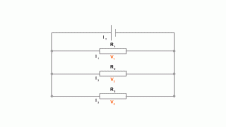 Resistors can be connected in a series. This results in various different p.d to be formed between each resisitor. The Voltage can be calculated as V = V1 + V2 + V3. Resistance in such a circuit is calculated by R = R1 + R2 + R3. To find the resistance the formula used is R = V/I. V is for Volts, R is for Resistance and I is for current. Resistors can also be connected in parallel circuits. The total resistance in this instance is calculated
Resistors can be connected in a series. This results in various different p.d to be formed between each resisitor. The Voltage can be calculated as V = V1 + V2 + V3. Resistance in such a circuit is calculated by R = R1 + R2 + R3. To find the resistance the formula used is R = V/I. V is for Volts, R is for Resistance and I is for current. Resistors can also be connected in parallel circuits. The total resistance in this instance is calculated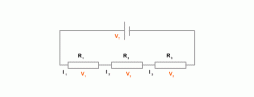
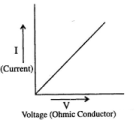 propertional to the p.d across it, if other factors such as temperature is constant. We can prove this by increasing the temperature of a circuit which has a ohmic conductor in it, as the temperature increases the resistance of the ohmic conductor also increases. If a bulb filament is heated its resistance will decrease showing that all semiconductor or insulator will lose its resistance once heated.
propertional to the p.d across it, if other factors such as temperature is constant. We can prove this by increasing the temperature of a circuit which has a ohmic conductor in it, as the temperature increases the resistance of the ohmic conductor also increases. If a bulb filament is heated its resistance will decrease showing that all semiconductor or insulator will lose its resistance once heated.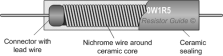 Resistors more commonly have a short length of carbon in them, which in some cases have a long wire winding around the ceramic rod in a casing. This makes it cheaper.
Resistors more commonly have a short length of carbon in them, which in some cases have a long wire winding around the ceramic rod in a casing. This makes it cheaper.
 The principle was founded by the French-Italian chemist Dr. Henry Louis Le Chatelier and the German physicist Nobel laureate Karl Ferdinand Braun. This principle is more
The principle was founded by the French-Italian chemist Dr. Henry Louis Le Chatelier and the German physicist Nobel laureate Karl Ferdinand Braun. This principle is more  commonly known as the Le Chatelier Braun principle. The principle states that when a chemical reaction is in the state of dynamic equilibrium, if the external factors such as temperature is not constant then the state of equilibrium will shift to the side which will try to prevent such a change.
commonly known as the Le Chatelier Braun principle. The principle states that when a chemical reaction is in the state of dynamic equilibrium, if the external factors such as temperature is not constant then the state of equilibrium will shift to the side which will try to prevent such a change.
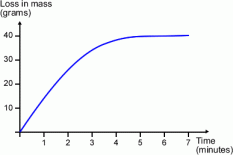 gain in concentration of products / time.
gain in concentration of products / time. 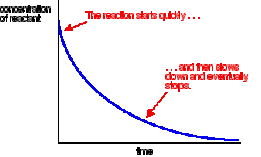 rate curve shows the loss of concentration of reactants over a certain period of time and may also show the amount of products formed over time. The factors to know about a rate curve is:
rate curve shows the loss of concentration of reactants over a certain period of time and may also show the amount of products formed over time. The factors to know about a rate curve is:
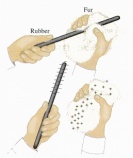 moving object. This force causes the formation of electricity which builds up in an insulator. If you walk on a nylon carpet and touch a door knob, you will feel a tingle on your finger, this is due to discharge of the electrons from your finger. Even in our atmosphere static electricity is produced. The warm and cold bodies of air pass through each other and due to the friction of their movement static electricity is formed, when it is formed in excess, it is then discharged in the form of lighting, this is also accompanied by an explosion we call thunder.
moving object. This force causes the formation of electricity which builds up in an insulator. If you walk on a nylon carpet and touch a door knob, you will feel a tingle on your finger, this is due to discharge of the electrons from your finger. Even in our atmosphere static electricity is produced. The warm and cold bodies of air pass through each other and due to the friction of their movement static electricity is formed, when it is formed in excess, it is then discharged in the form of lighting, this is also accompanied by an explosion we call thunder.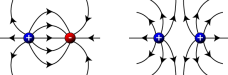 charged object from earth. When a negatively charged object is touched, the electrons flow from the object and into the earth. This is a simple method of discharging an object.
charged object from earth. When a negatively charged object is touched, the electrons flow from the object and into the earth. This is a simple method of discharging an object.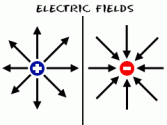 A positive charge has currents flowing outwards, while a negative charge has current flowing towards it. If you put two objects which have the same charge the two charges will repel each other but the charges which are opposite will attract each other.
A positive charge has currents flowing outwards, while a negative charge has current flowing towards it. If you put two objects which have the same charge the two charges will repel each other but the charges which are opposite will attract each other. In the 16th century, the famous physicist Dr. William Gilbert, then observed this property much more closely.
In the 16th century, the famous physicist Dr. William Gilbert, then observed this property much more closely.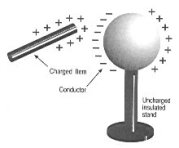 side where the ruler is and the concentration of the negative charge is increased on one side of the paper while on the other side the positive charge is in higher concentration. This causes attraction between the two things. This whole process is called electromagnetic induction.
side where the ruler is and the concentration of the negative charge is increased on one side of the paper while on the other side the positive charge is in higher concentration. This causes attraction between the two things. This whole process is called electromagnetic induction.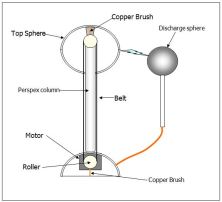 charge itself. It is charged by a rubber rubbing off on a perspex bar. If you touch it while standing on an insulator, your body will be charged.
charge itself. It is charged by a rubber rubbing off on a perspex bar. If you touch it while standing on an insulator, your body will be charged. each other. There is a smaller sphere near the van der Graaf which is connected to the earth, it will take away the electrons of the Van der Graaf.
each other. There is a smaller sphere near the van der Graaf which is connected to the earth, it will take away the electrons of the Van der Graaf.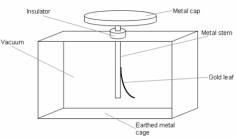 using the same principle as the electrostatic induction.
using the same principle as the electrostatic induction.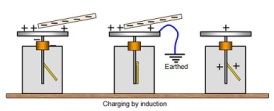

 would always point to the same direction. At the time no one had the idea that the rock actually had a magnetic property in it.
would always point to the same direction. At the time no one had the idea that the rock actually had a magnetic property in it.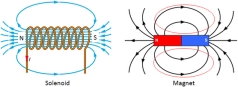 The field lines always come from the North pole of a magnet, and to the south pole of the magnet. The picture other than the magnet you see is a solenoid which will be discussed later in this article.
The field lines always come from the North pole of a magnet, and to the south pole of the magnet. The picture other than the magnet you see is a solenoid which will be discussed later in this article.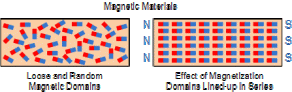 that it will be directed towards the North pole of the magnet. This causes the formation of magnets.
that it will be directed towards the North pole of the magnet. This causes the formation of magnets.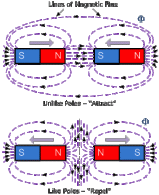 The reason behind this is that the field lines come out of the North pole while it is taken in by the South pole, causing the two to be attracted while if you place two south poles, field lines are deflected and a null point is formed in the center.
The reason behind this is that the field lines come out of the North pole while it is taken in by the South pole, causing the two to be attracted while if you place two south poles, field lines are deflected and a null point is formed in the center.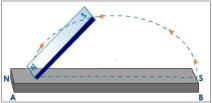 are three ways for magnetising. One method is to use a single touch method. In this method, we stroke a straight iron bar with a magnet several times. If the North pole is used, the side that is first touched by the magnet then it will become the North pole while the other side will become the South pole.
are three ways for magnetising. One method is to use a single touch method. In this method, we stroke a straight iron bar with a magnet several times. If the North pole is used, the side that is first touched by the magnet then it will become the North pole while the other side will become the South pole. 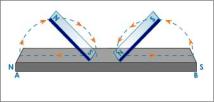
 take the opposite polarity that the pole used to stroke it.
take the opposite polarity that the pole used to stroke it.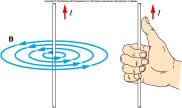
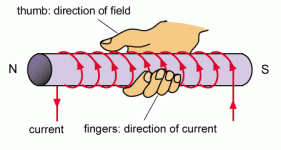
 note is that when a solenoid has a current passing through it, the solenoid will have the same magnetic field as a bar magnet. When a wire moves around in a circle through a cardboard, there will be two magnetic fields formed. The first will move anticlockwise and the other clockwise.
note is that when a solenoid has a current passing through it, the solenoid will have the same magnetic field as a bar magnet. When a wire moves around in a circle through a cardboard, there will be two magnetic fields formed. The first will move anticlockwise and the other clockwise.
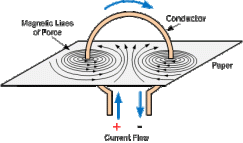
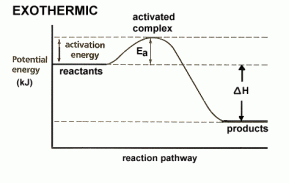 .
.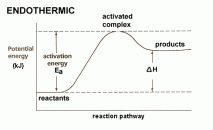 denoted by ΔH. Enthalpy is always negative in case of an exothermic reaction because energy is given off as heat energy and will always be positive in case of an endothermic reaction because energy is always taken in from the surroundings as heat energy.
denoted by ΔH. Enthalpy is always negative in case of an exothermic reaction because energy is given off as heat energy and will always be positive in case of an endothermic reaction because energy is always taken in from the surroundings as heat energy.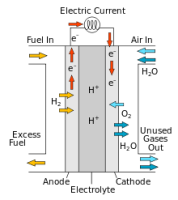 into it from one side and on the other side oxygen gas is passed in as an oxidising agent. There is also a catalyst present which helps with oxidation of hydrogen. The cell has two electrodes in it with the electrolysis. Hydrogen turns into cations at the anode and moves to the cathode, then reacts with the electrons and with oxygen to form water. This whole process results in a charge being formed that is passed along the circuit.
into it from one side and on the other side oxygen gas is passed in as an oxidising agent. There is also a catalyst present which helps with oxidation of hydrogen. The cell has two electrodes in it with the electrolysis. Hydrogen turns into cations at the anode and moves to the cathode, then reacts with the electrons and with oxygen to form water. This whole process results in a charge being formed that is passed along the circuit.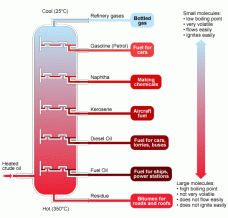 oil have different boiling points ranging from 40°C to 360°C. The column is heated at different temperatures causing the liquid to turn into gas and pass into a condensing tube where it condenses into gas. The products are diesel, LPG, gasoline, petroleum, kerosene.
oil have different boiling points ranging from 40°C to 360°C. The column is heated at different temperatures causing the liquid to turn into gas and pass into a condensing tube where it condenses into gas. The products are diesel, LPG, gasoline, petroleum, kerosene.

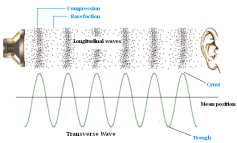 causes the air particles to vibrate as well causing the air particles to form rarefactions and compressions. A compression is an area in which particles are squashes together causing an area of high pressure. A rarefaction is an area in which particles are further apart from each other. This creates an area of low pressure. The wavelength of the wave is found by measuring the length from the middle of a compression to the other compression or from the middle of a rarefaction to the other rarefaction.
causes the air particles to vibrate as well causing the air particles to form rarefactions and compressions. A compression is an area in which particles are squashes together causing an area of high pressure. A rarefaction is an area in which particles are further apart from each other. This creates an area of low pressure. The wavelength of the wave is found by measuring the length from the middle of a compression to the other compression or from the middle of a rarefaction to the other rarefaction.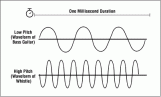 The pitch of a sound wave depends upon the frequency of a wave or more accurately the wavelength of the wave. The shorther the wavelength the higher the pitch and the longer the wavelength the smaller the pitch.
The pitch of a sound wave depends upon the frequency of a wave or more accurately the wavelength of the wave. The shorther the wavelength the higher the pitch and the longer the wavelength the smaller the pitch.
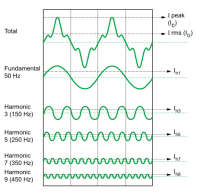 Even if the frequency and the amplitude are same the sound will always be different. This is because these instruments produce a whole complex waveform. Each waveform contains a set of waves called harmonics or sometimes also known as an overtone (plural for harmonic). Each harmonic has different frequency, the first harmonic tends to have the lowest frequency and the frequency increases with the next harmonic.
Even if the frequency and the amplitude are same the sound will always be different. This is because these instruments produce a whole complex waveform. Each waveform contains a set of waves called harmonics or sometimes also known as an overtone (plural for harmonic). Each harmonic has different frequency, the first harmonic tends to have the lowest frequency and the frequency increases with the next harmonic.
 und. Ultrasound has a lot of uses. One of its most common uses includes prenatal dioagnosis. A handheld device is used to emit ultrasound waves, these waves are then reflected back when it crosses the boundries between the different tissues of the mother and the fetus. The waves are then recepted by the device and then sent to the computer to form an image.
und. Ultrasound has a lot of uses. One of its most common uses includes prenatal dioagnosis. A handheld device is used to emit ultrasound waves, these waves are then reflected back when it crosses the boundries between the different tissues of the mother and the fetus. The waves are then recepted by the device and then sent to the computer to form an image.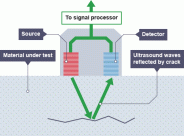

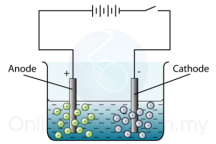
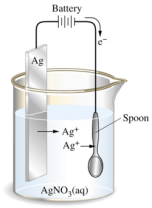 The object which needs to be electroplated is first dipped in acid to remove any traces of oxides. This object is then made the cathode and the metal needed to cover it is made the anode.
The object which needs to be electroplated is first dipped in acid to remove any traces of oxides. This object is then made the cathode and the metal needed to cover it is made the anode.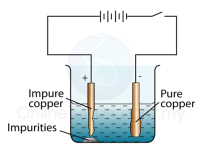 and the copper (II) sulphide is used as the electrolyte.
and the copper (II) sulphide is used as the electrolyte.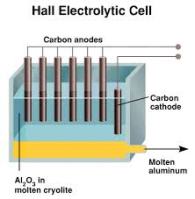 The mixture is then poured in a Hall-Héroult, and then heated. Inert graphite electrodes are used in this case. At the cathode oxygen gas is given off which combines with the carbon (since graphite is an allotrope of carbon). This forms the carbon dioxide gas. The molten aluminum then seperates from the cryolite and then drains off.
The mixture is then poured in a Hall-Héroult, and then heated. Inert graphite electrodes are used in this case. At the cathode oxygen gas is given off which combines with the carbon (since graphite is an allotrope of carbon). This forms the carbon dioxide gas. The molten aluminum then seperates from the cryolite and then drains off.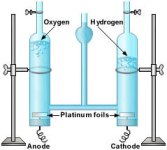 have any ions. Inert platinum electrodes are used for electrolysis.
have any ions. Inert platinum electrodes are used for electrolysis.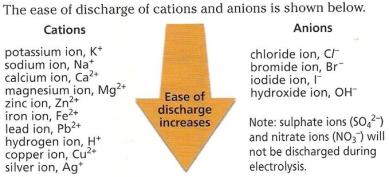

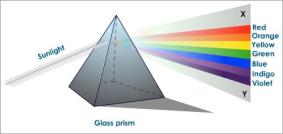
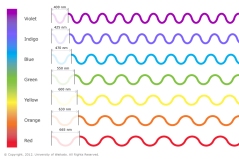
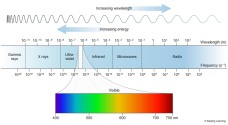 The rays of light are also in a continuous spectrum as in the case of visible light. The Gamma ray has the shortest wavelength but the highest frequency while the radiowaves have the longest wavelength but have shortest frequency.
The rays of light are also in a continuous spectrum as in the case of visible light. The Gamma ray has the shortest wavelength but the highest frequency while the radiowaves have the longest wavelength but have shortest frequency.
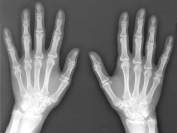 The X rays are also used in the medicinal sectore, these are also capable to penetrate human skin but are stopped at the bones which are much harder than human flesh. This is useful to make Xray diagrams of bones to detect injury.
The X rays are also used in the medicinal sectore, these are also capable to penetrate human skin but are stopped at the bones which are much harder than human flesh. This is useful to make Xray diagrams of bones to detect injury. produce more melanin.
produce more melanin. of wavelengths and frequencies. It is used in many household applications such as alarm detectors which detect a change in heat energy which is detected by these rays. These rays are also used by your t.v remote which sends signals to the t.v, each button has its own frequency and a little difference in wavelength in the infra-red waves.
of wavelengths and frequencies. It is used in many household applications such as alarm detectors which detect a change in heat energy which is detected by these rays. These rays are also used by your t.v remote which sends signals to the t.v, each button has its own frequency and a little difference in wavelength in the infra-red waves. 
 Radio waves are reflected by the Earth’s atmosphere, this is why these are used in communications. The waves can travel over the horizon, to large distances making it useful for contacting someone across the country.
Radio waves are reflected by the Earth’s atmosphere, this is why these are used in communications. The waves can travel over the horizon, to large distances making it useful for contacting someone across the country.
 ht waves follow the same principle as any other wave. When a few parallel light waves strike a smooth, flat surface, it will change direction and move away from the surface, in parallel rays. This is the principle of reflection.
ht waves follow the same principle as any other wave. When a few parallel light waves strike a smooth, flat surface, it will change direction and move away from the surface, in parallel rays. This is the principle of reflection.
 the wall, and add the tape, then turn on the projector, it will form an image on the wall.
the wall, and add the tape, then turn on the projector, it will form an image on the wall. the medium light is originally travelling in, the light wave slows down and then changes its direction. This change in direction is called refraction.
the medium light is originally travelling in, the light wave slows down and then changes its direction. This change in direction is called refraction. There comes a point when the angle of refraction becomes 90°. At this point the angle of incidence is called the critical angle.
There comes a point when the angle of refraction becomes 90°. At this point the angle of incidence is called the critical angle. the lens axis parallel to the principle axis and then from the lens axis change its direction towards the principle axis.
the lens axis parallel to the principle axis and then from the lens axis change its direction towards the principle axis.
 is at the focal point, the image formed will be of the same size as the object.
is at the focal point, the image formed will be of the same size as the object.
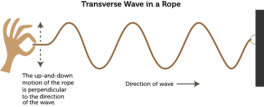 moving from their positions but vibrating, transferring their kinetic energy to the other particle. If you tie a rope to a wall and grab the other end and move it up and down continuously, you would see a wave produce on the rope while the rope itself does not move towards the wall even though the kinetic energy is being supplied to the wall.
moving from their positions but vibrating, transferring their kinetic energy to the other particle. If you tie a rope to a wall and grab the other end and move it up and down continuously, you would see a wave produce on the rope while the rope itself does not move towards the wall even though the kinetic energy is being supplied to the wall. of wave is if you attach a spring with a wall and then grab the other end and move the spring forward and backward continuously. You will notice a series of compression and rarefaction formed in the spring which are properties of the longitudinal wave.
of wave is if you attach a spring with a wall and then grab the other end and move the spring forward and backward continuously. You will notice a series of compression and rarefaction formed in the spring which are properties of the longitudinal wave.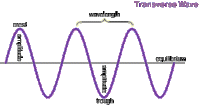
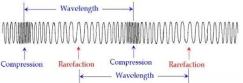 a longitudinal wave the wavelength is calculated by measuring the distance from the middle of a compression to the adjacent middle of the compression or from the middle of the rarefaction to adjacent middle of the rarefaction.
a longitudinal wave the wavelength is calculated by measuring the distance from the middle of a compression to the adjacent middle of the compression or from the middle of the rarefaction to adjacent middle of the rarefaction.

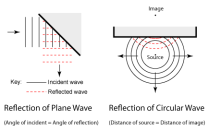 seen in ripple waves. If these waves touch a straight wall which is at an angle from the waves, the waves would then reflect to another direction. An imaginary straight line is made at an from the point at which the waves touch the wall, this is called the normal, the angle formed from the normal and the direction of the waves is called an angle of incidence. The angle formed from the normal and the point of reflection is called the angle of reflection.
seen in ripple waves. If these waves touch a straight wall which is at an angle from the waves, the waves would then reflect to another direction. An imaginary straight line is made at an from the point at which the waves touch the wall, this is called the normal, the angle formed from the normal and the direction of the waves is called an angle of incidence. The angle formed from the normal and the point of reflection is called the angle of reflection.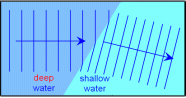 taken from the ripple waves. If a wave moves from deeper water to shallower water then the wave slows down and then refracts. This is because when the front part of the wave touches the shallower water it slows down, and then the rest of the wave follows up and slows down as well and this continues on while the wave refracts.
taken from the ripple waves. If a wave moves from deeper water to shallower water then the wave slows down and then refracts. This is because when the front part of the wave touches the shallower water it slows down, and then the rest of the wave follows up and slows down as well and this continues on while the wave refracts.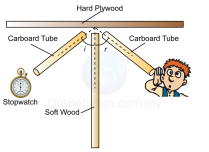 suggested. Place a hard flat surface. Place a clock with a wide tube reaching towards the flat surface. Then place another tube at the adjacent side of the other tube and listen through the tube. You should hear the clock ticking.
suggested. Place a hard flat surface. Place a clock with a wide tube reaching towards the flat surface. Then place another tube at the adjacent side of the other tube and listen through the tube. You should hear the clock ticking.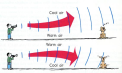 refraction happens in between the layers in the atmosphere. During day time the ground air becomes warmer while the air high up is colder which is why sound waves refract up wards. At night the air in ground is cold while the air high up is warmer causing the sound waves to refract downwards.
refraction happens in between the layers in the atmosphere. During day time the ground air becomes warmer while the air high up is colder which is why sound waves refract up wards. At night the air in ground is cold while the air high up is warmer causing the sound waves to refract downwards.
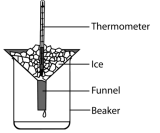
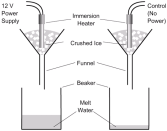 energy to a pure solid and calculating the mass of the liquid formed. For example, a known mass of pure ice is placed in a funnel with a beaker under the funnel. When water starts to drop out of the funnel, the temperature of the ice can be taken 0°C. Then an electric heater is placed inside the crushed ice and a known amount of energy is provided. Since the heater is provided with electrical energy, it can be calculated efficiently. When the ice is completely converted into water then the mass of water is calculated using an electronic balance.
energy to a pure solid and calculating the mass of the liquid formed. For example, a known mass of pure ice is placed in a funnel with a beaker under the funnel. When water starts to drop out of the funnel, the temperature of the ice can be taken 0°C. Then an electric heater is placed inside the crushed ice and a known amount of energy is provided. Since the heater is provided with electrical energy, it can be calculated efficiently. When the ice is completely converted into water then the mass of water is calculated using an electronic balance.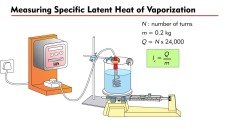 heater and then find the mass lost from the initial mass of the liquid. For example; A beaker is placed on an electronic balance, it is filled with pure water and its mass is calculated excluding the tare value. An electric heater is placed and the substance is heated. Then we measure the loss of mass of the liquid over the amount of power used to heat the substance. Note that the loss of mass of the liquid is a gain in mass of gas, such as loss of 3g of liquid is an increase in 3 gram of gas.
heater and then find the mass lost from the initial mass of the liquid. For example; A beaker is placed on an electronic balance, it is filled with pure water and its mass is calculated excluding the tare value. An electric heater is placed and the substance is heated. Then we measure the loss of mass of the liquid over the amount of power used to heat the substance. Note that the loss of mass of the liquid is a gain in mass of gas, such as loss of 3g of liquid is an increase in 3 gram of gas.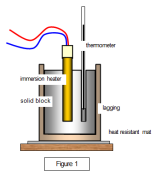 with a thermometer. The initial temperature is calculated. The solid is then provided with an insulation to avoid any and loss of energy. The electric heater then provides the solid with a specific amount of energy. The rise in temperature is then calculated to find the heat capacity of the solid.
with a thermometer. The initial temperature is calculated. The solid is then provided with an insulation to avoid any and loss of energy. The electric heater then provides the solid with a specific amount of energy. The rise in temperature is then calculated to find the heat capacity of the solid.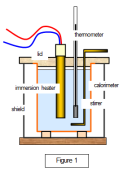 finding the heat capacity of a liquid, the liquid is poured in a container and is capped with a copper calorimeter. The initial temperature is measured by a thermometer. Then the liquid is heated with an electric heater and then the rise in temperature is measured. The problem with this is that the copper calorimeter absorbs some of the energy causing a little problem with measuring heat capacity.
finding the heat capacity of a liquid, the liquid is poured in a container and is capped with a copper calorimeter. The initial temperature is measured by a thermometer. Then the liquid is heated with an electric heater and then the rise in temperature is measured. The problem with this is that the copper calorimeter absorbs some of the energy causing a little problem with measuring heat capacity.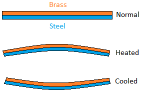 metals are best at this because these are good conductors of heat. The heat energy causes the metals to expand. This is why metal bridges tend to have rollers on one of its end to allow the bridge to expand a bit. A bimetal strip has two different types of metals, with one that expands sooner than the other causing the strip to bend.
metals are best at this because these are good conductors of heat. The heat energy causes the metals to expand. This is why metal bridges tend to have rollers on one of its end to allow the bridge to expand a bit. A bimetal strip has two different types of metals, with one that expands sooner than the other causing the strip to bend.
 Compounds are also made by chemical processes compared to mixtures. Compounds are written as the combination of the atomic symbol of the elements such as Sodium Chloride has the atomic symbol NaCl. Mixtures are not given atomic symbols.
Compounds are also made by chemical processes compared to mixtures. Compounds are written as the combination of the atomic symbol of the elements such as Sodium Chloride has the atomic symbol NaCl. Mixtures are not given atomic symbols.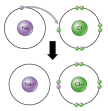 electron and the atom which wants to gain them. The atom which loses the electrons gains a positive charge due to increased number of protons than electrons which produces a cation.
electron and the atom which wants to gain them. The atom which loses the electrons gains a positive charge due to increased number of protons than electrons which produces a cation.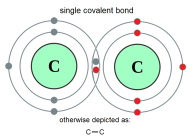 way mainly because the atoms can not produce sufficient energy for ionic bonding. This why these atoms form covalent bonds. These bonds are represented in words as Cl-Cl, O=O, N≡N etc. These bonds are a result of strong electrostatic forces between the valence electrons and the positively charge nuclei of the atom.
way mainly because the atoms can not produce sufficient energy for ionic bonding. This why these atoms form covalent bonds. These bonds are represented in words as Cl-Cl, O=O, N≡N etc. These bonds are a result of strong electrostatic forces between the valence electrons and the positively charge nuclei of the atom.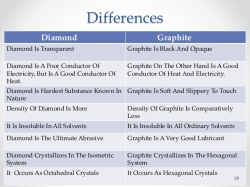 Graphite is also composed of carbon. Each carbon atoms are bonded with 3 other carbon atoms. The atoms form a hexagon. There are valence electrons available in graphite causing it to be a semi conductor. Both diamond and graphite have a very high melting point but other wise each have different properties. Graphite has weak intermolecular bonds thus it can be used as a lubricant whether a solid or mixed in a solvent. It is also used in pencil which is not composed of lead at all. Graphite is comparatively softer material than diamond.
Graphite is also composed of carbon. Each carbon atoms are bonded with 3 other carbon atoms. The atoms form a hexagon. There are valence electrons available in graphite causing it to be a semi conductor. Both diamond and graphite have a very high melting point but other wise each have different properties. Graphite has weak intermolecular bonds thus it can be used as a lubricant whether a solid or mixed in a solvent. It is also used in pencil which is not composed of lead at all. Graphite is comparatively softer material than diamond. strong bond between the atoms. The valence electrons also result in the metal being a very good conductor.
strong bond between the atoms. The valence electrons also result in the metal being a very good conductor.
 he melting point of a pure substance. A pure substance is essential for this because impurities in a substance alters the boiling point and the melting point of the substance. These impurities lowers the melting point and raise the boiling point.
he melting point of a pure substance. A pure substance is essential for this because impurities in a substance alters the boiling point and the melting point of the substance. These impurities lowers the melting point and raise the boiling point.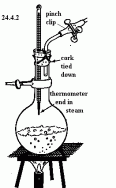 the temperature and mark this as the melting point of the pure substance.
the temperature and mark this as the melting point of the pure substance. liquid in glass thermometer has a few properties which is very helpful for measuring temperature. The glass which makes the body of the capillary tube is thick acting as a magnifying glass for easy reading of the measurements. The bore was first made of glass but commonly is made of metal since metals are good conductors of heat. The
liquid in glass thermometer has a few properties which is very helpful for measuring temperature. The glass which makes the body of the capillary tube is thick acting as a magnifying glass for easy reading of the measurements. The bore was first made of glass but commonly is made of metal since metals are good conductors of heat. The  liquid inside the glass is either alcohol (ethanol) or mercury. Both have their disadvantages and advantages but mercury is more commonly used as it reacts to heat better than alcohol.
liquid inside the glass is either alcohol (ethanol) or mercury. Both have their disadvantages and advantages but mercury is more commonly used as it reacts to heat better than alcohol.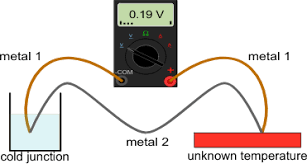 electrical device. The advantages for using this kind of thermometer is:
electrical device. The advantages for using this kind of thermometer is: Thermochromic or Thermocolor thermometer is more simple to use and is used for household purposes, mainly to check the temperature of an ill person. The thermometer has liquid crystals in it which change color due to rise in temperature.
Thermochromic or Thermocolor thermometer is more simple to use and is used for household purposes, mainly to check the temperature of an ill person. The thermometer has liquid crystals in it which change color due to rise in temperature.
 lower the temperature of an object until the temperature of the substance is equal to that of its environment.
lower the temperature of an object until the temperature of the substance is equal to that of its environment.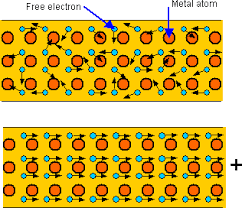 Metals tend to be the best at conducting heat and electricity. This is because of their structure. A metal has ions in its long matrix of atoms and ions. There are some electrons in metals that are called delocalized or free because they are not held by an ion. These electrons move around the metal structure freely. When metals are heated, the particles do vibrate but the free electrons move around the matrix of ions and atoms, these electrons spread the heat energy across the metal causing the metal to heat up faster than non metals.
Metals tend to be the best at conducting heat and electricity. This is because of their structure. A metal has ions in its long matrix of atoms and ions. There are some electrons in metals that are called delocalized or free because they are not held by an ion. These electrons move around the metal structure freely. When metals are heated, the particles do vibrate but the free electrons move around the matrix of ions and atoms, these electrons spread the heat energy across the metal causing the metal to heat up faster than non metals.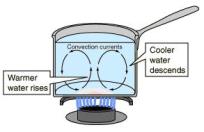 corner in the liquid. Heat up the beaker over the Bunsen burner and notice as the dye diffuses into the liquid. The dye moves up and slowly moves down again. This is because the bottom the beaker is heated, the liquid in that region gains heat energy and loses its density, therefore it travels to the upper region, where the temperature is still low. The water from the top moves down to the hot region and gains heat energy. This movement allows heat to be transferred to the whole liquid until the liquid starts to boil and change its state to gas.
corner in the liquid. Heat up the beaker over the Bunsen burner and notice as the dye diffuses into the liquid. The dye moves up and slowly moves down again. This is because the bottom the beaker is heated, the liquid in that region gains heat energy and loses its density, therefore it travels to the upper region, where the temperature is still low. The water from the top moves down to the hot region and gains heat energy. This movement allows heat to be transferred to the whole liquid until the liquid starts to boil and change its state to gas. forms the sea breeze
forms the sea breeze vacuum between the Earth and the Sun but heat is still transferred to the Earth. This is because heat is transferred in the form of infra-red rays along with other rays emitted by the Sun. These infra red rays have short wavelength and is absorbed by the Earth. The soil absorbs the rays and emits them back to the atmosphere. This time the rays have a longer wavelength. These rays are absorbed by the greenhouse gases. The rays are again send back to Earth causing the Earth to heat up. This maintains the temperature of the Earth.
vacuum between the Earth and the Sun but heat is still transferred to the Earth. This is because heat is transferred in the form of infra-red rays along with other rays emitted by the Sun. These infra red rays have short wavelength and is absorbed by the Earth. The soil absorbs the rays and emits them back to the atmosphere. This time the rays have a longer wavelength. These rays are absorbed by the greenhouse gases. The rays are again send back to Earth causing the Earth to heat up. This maintains the temperature of the Earth.
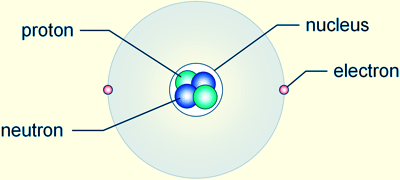 negatively charged subatomic particles which are found in the shells of the atom. The neutrons and protons are found in the nucleus of the atoms. Protons have a positive charge while neutrons carry no charge. Since atoms are not charged particles, that means that the number of protons and electrons are same.
negatively charged subatomic particles which are found in the shells of the atom. The neutrons and protons are found in the nucleus of the atoms. Protons have a positive charge while neutrons carry no charge. Since atoms are not charged particles, that means that the number of protons and electrons are same.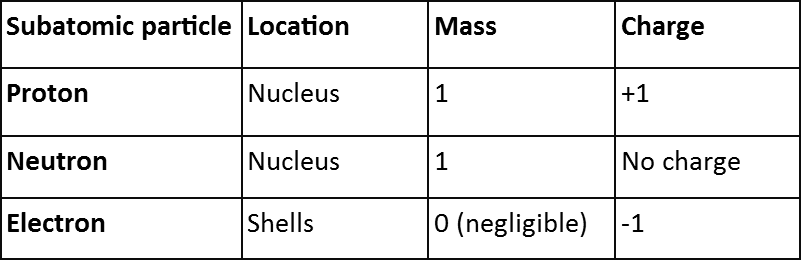 The mass of protons and neutrons are greater than electrons. If the mass of proton is 1 then the mass of electron is taken 0. Even though these two subatomic particles are different in masses they carry a same charge.
The mass of protons and neutrons are greater than electrons. If the mass of proton is 1 then the mass of electron is taken 0. Even though these two subatomic particles are different in masses they carry a same charge.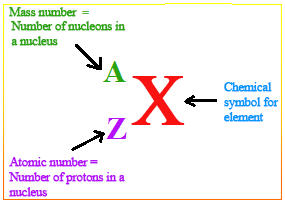
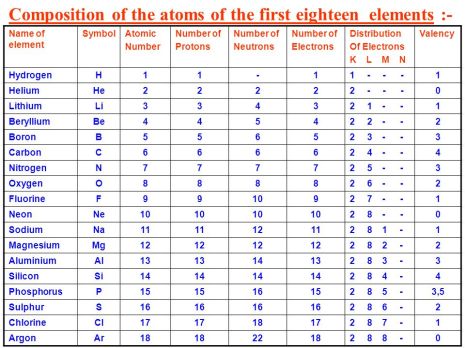 duplet. The 2nd shell holds 8 electrons forming an octect. The 3rd can hold 18 and the fourth can hold up to 32. If these limits are crossed then the electrons are to be placed in the next shell. However calcium and potassium are free of such pattern because as their 3rd shells exceed 8 electrons it is placed in another shell.
duplet. The 2nd shell holds 8 electrons forming an octect. The 3rd can hold 18 and the fourth can hold up to 32. If these limits are crossed then the electrons are to be placed in the next shell. However calcium and potassium are free of such pattern because as their 3rd shells exceed 8 electrons it is placed in another shell.
 transparent but because it condenses, as it comes out of the kettle into the low temperature environment, it forms small clouds.
transparent but because it condenses, as it comes out of the kettle into the low temperature environment, it forms small clouds. other to transfer or gain energy. This is the case with liquids and gases. This is the reason the solids:
other to transfer or gain energy. This is the case with liquids and gases. This is the reason the solids: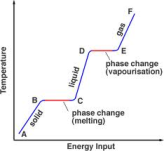

 all objects. This supports the fact that the heavier an object is and the higher it is raised above the ground the greater the amount of gravitational potential energy it contains. When it falls to the ground, it loses its gravitational potential energy. This can be shown through the equation:
all objects. This supports the fact that the heavier an object is and the higher it is raised above the ground the greater the amount of gravitational potential energy it contains. When it falls to the ground, it loses its gravitational potential energy. This can be shown through the equation: Potential energy can be stored in some ways. One type of potential energy is chemical energy. Energy is stored and is slowly utilized when a group of atoms or molecules group together to convert the energy into other form. The energy can be utilized much more quickly depending on the source of energy, such as, a fossil fuel converts its chemical energy into heat energy very quickly when burned.
Potential energy can be stored in some ways. One type of potential energy is chemical energy. Energy is stored and is slowly utilized when a group of atoms or molecules group together to convert the energy into other form. The energy can be utilized much more quickly depending on the source of energy, such as, a fossil fuel converts its chemical energy into heat energy very quickly when burned.
 energy slowly and it gradually falls until its velocity doubles.
energy slowly and it gradually falls until its velocity doubles. sources of energy which might not be much helpful as the fossil fuels are.
sources of energy which might not be much helpful as the fossil fuels are. solar panels. The dams and barriers formed on a river makes an artificial lake. The water flows through the pipes in the dams and turns the turbines forming electricity. The water is also used as a source of energy in tidal form of power generation. There is a barrier formed between two tides. The water from the higher tide is flowed through a pipe containing a turbine. The turbine is moved causing the electrical energy to be formed and the water flows into the lower tide. Wind mills are used for utilizing wind. The kinetic energy from wind moves the turbine from the wind mills producing electrical energy.
solar panels. The dams and barriers formed on a river makes an artificial lake. The water flows through the pipes in the dams and turns the turbines forming electricity. The water is also used as a source of energy in tidal form of power generation. There is a barrier formed between two tides. The water from the higher tide is flowed through a pipe containing a turbine. The turbine is moved causing the electrical energy to be formed and the water flows into the lower tide. Wind mills are used for utilizing wind. The kinetic energy from wind moves the turbine from the wind mills producing electrical energy.
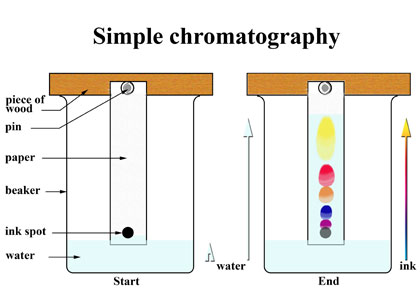 upwards in the dish. The solvent should not touch the ink as it will dissolve in the solvent. As the solvent moves up the paper, the different pigments will get dissolved with the solvent and move up the chromatography paper. As the solvent reaches the top of the paper the pigments of the ink will stop at a certain point. This is a good way of showing the amount of pigments in an ink. The separated pigments will then be called a chromatogram. You will need to measure the Rf value by the equation;
upwards in the dish. The solvent should not touch the ink as it will dissolve in the solvent. As the solvent moves up the paper, the different pigments will get dissolved with the solvent and move up the chromatography paper. As the solvent reaches the top of the paper the pigments of the ink will stop at a certain point. This is a good way of showing the amount of pigments in an ink. The separated pigments will then be called a chromatogram. You will need to measure the Rf value by the equation;
 expressed in the SI units of Kilogram (Kg) for large masses while for small masses gram (g) is used. For even smaller masses milligram (mg) may also be used. The mass of 1 Kg is equal to a 1000 grams will 1 gram is equal to a 1000 milligrams.
expressed in the SI units of Kilogram (Kg) for large masses while for small masses gram (g) is used. For even smaller masses milligram (mg) may also be used. The mass of 1 Kg is equal to a 1000 grams will 1 gram is equal to a 1000 milligrams. horizontally. The scale is then read. The use of a beam balance requires skills of the user and is dependent on it for accurate results. It does not require a power source.
horizontally. The scale is then read. The use of a beam balance requires skills of the user and is dependent on it for accurate results. It does not require a power source. I units cubic decimeter (dm^3) for large volumes while for smaller volumes cubic centimeter (cm^3) is used. Cubic meter (m^3) is also used when measuring large volumes. Liter and milliliter are used sometimes but are not agreed SI units to be used for calculating volume so they are converted to cubic decimeter.
I units cubic decimeter (dm^3) for large volumes while for smaller volumes cubic centimeter (cm^3) is used. Cubic meter (m^3) is also used when measuring large volumes. Liter and milliliter are used sometimes but are not agreed SI units to be used for calculating volume so they are converted to cubic decimeter. two types of apparatus used for measuring volumes. There are those apparatus which are used to calculate accurate measurements and there are those which are used to calculate approximate measurement. The beaker is used for approximate measurement of volume of substance but the burrete is used for accurate measurement. For measuring volumes of gas, the gas syringe is used while the burette can also be used for this purpose by inverting it in a container filled with water.
two types of apparatus used for measuring volumes. There are those apparatus which are used to calculate accurate measurements and there are those which are used to calculate approximate measurement. The beaker is used for approximate measurement of volume of substance but the burrete is used for accurate measurement. For measuring volumes of gas, the gas syringe is used while the burette can also be used for this purpose by inverting it in a container filled with water. Time is measured in minutes (m) and seconds (s) by using watch. A digital stopwatch is used for best measurements. Time is very important to ensure the accuracy of many experiments.
Time is measured in minutes (m) and seconds (s) by using watch. A digital stopwatch is used for best measurements. Time is very important to ensure the accuracy of many experiments. mercury is used. In case of alcohol the measurement is taken from the bottom of the meniscus. The mercury is used more commonly in thermometers because it reacts towards temperature more fast than alcohol.
mercury is used. In case of alcohol the measurement is taken from the bottom of the meniscus. The mercury is used more commonly in thermometers because it reacts towards temperature more fast than alcohol. 

 area of the boots touching the ground. The snow shoes which have a larger area touching the snow causes the exertion of low amount of pressure.
area of the boots touching the ground. The snow shoes which have a larger area touching the snow causes the exertion of low amount of pressure. liquid is calculated by the equation:
liquid is calculated by the equation: length of the oil and the water in the opposite of the oil and use their densities in the equation:
length of the oil and the water in the opposite of the oil and use their densities in the equation: are distant from each other. They also collide with each other and exert a force on each other to move away from each other. If the air is contained in a jar the particles of the gas will collide with the walls of the jar. This forms a pressure. This can be tested by a manometer. The manometer is just like a U tube with a liquid in it. Air is blown into the manometer from one side and the pressure by the air particles causes the liquid to move down the tube on the side where air is blown but rises on the other side of the manometer.
are distant from each other. They also collide with each other and exert a force on each other to move away from each other. If the air is contained in a jar the particles of the gas will collide with the walls of the jar. This forms a pressure. This can be tested by a manometer. The manometer is just like a U tube with a liquid in it. Air is blown into the manometer from one side and the pressure by the air particles causes the liquid to move down the tube on the side where air is blown but rises on the other side of the manometer.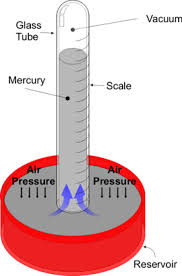 sea level, the atmospheric pressure decreases. The atmospheric pressure is expressed in the units; Atmosphere (atm), Pascals and Kilopascals (Pa and kPa), and in millimeters. The atmospheric pressure is measured by a barometer. This is sometimes called a torricilian in respect to its inventor Torreceli. The barometer is filled with mercury and is placed inverted on a trough filled with mercury. The cylinder is stood still by the atmospheric pressure. Measure the column of the mercury to calculate the atmospheric pressure in millimeters. The atmospheric pressure is not constant and can change.
sea level, the atmospheric pressure decreases. The atmospheric pressure is expressed in the units; Atmosphere (atm), Pascals and Kilopascals (Pa and kPa), and in millimeters. The atmospheric pressure is measured by a barometer. This is sometimes called a torricilian in respect to its inventor Torreceli. The barometer is filled with mercury and is placed inverted on a trough filled with mercury. The cylinder is stood still by the atmospheric pressure. Measure the column of the mercury to calculate the atmospheric pressure in millimeters. The atmospheric pressure is not constant and can change. cted to a container filled with a gas. If the gas particles have low volume in the large container then it’s pressure may be equal to atmospheric pressure. This causes the liquid in the legs of the manometer to not loosen its height. If the gas is present in large volume, it will exert more pressure than the atmospheric air and push the liquid down the leg causing a decrease in the height of the column of liquid in one leg of the manometer but increases the height of the column of liquid in the other leg.
cted to a container filled with a gas. If the gas particles have low volume in the large container then it’s pressure may be equal to atmospheric pressure. This causes the liquid in the legs of the manometer to not loosen its height. If the gas is present in large volume, it will exert more pressure than the atmospheric air and push the liquid down the leg causing a decrease in the height of the column of liquid in one leg of the manometer but increases the height of the column of liquid in the other leg.
 test with a food just crush, grind and ground the food in water.
test with a food just crush, grind and ground the food in water.
 Add the food in a dry boiling tube. You can use cheese or oil or any other lipid.
Add the food in a dry boiling tube. You can use cheese or oil or any other lipid. contains the protein albumin.
contains the protein albumin.
 of restriction endonucleases. Restriction endonucleases are also known as restriction enzymes. These enzymes cut off the part of the plasmid.
of restriction endonucleases. Restriction endonucleases are also known as restriction enzymes. These enzymes cut off the part of the plasmid. engineering. The genetically modified bacteria will release its plasmids in to the cells and that part can be cut off and can be grown into a new GM plant by micropropagation.
engineering. The genetically modified bacteria will release its plasmids in to the cells and that part can be cut off and can be grown into a new GM plant by micropropagation.


 glucose in the blood. This raises the blood glucose level and causes diabetes in children. Insulin is provided to the patient by injections regularly.
glucose in the blood. This raises the blood glucose level and causes diabetes in children. Insulin is provided to the patient by injections regularly. food with carbohydrates in it. Keeping a healthy lifestyle prevents diabetes in the first place, however due to the gene which causes diabetes we cannot do anything to prevent it but we can treat it. In case of type 1 diabetes and in severe cases of type 2 diabetes, insulin injections are necessary to be taken every day. We cannot take insulin as tablets because it is a protein and will be digested in the stomach. To determine the use of insulin patients should use the biosensor which uses a test strip to determine blood glucose concentration. The patient must pierce his/her skin and rub the blood on to the test strip and place it in the biosensor. The device is very accurate and will calculate the exact amount of glucose concentration. This determines whether a patient needs insulin injections or not. A patient will have to test this several times a day.
food with carbohydrates in it. Keeping a healthy lifestyle prevents diabetes in the first place, however due to the gene which causes diabetes we cannot do anything to prevent it but we can treat it. In case of type 1 diabetes and in severe cases of type 2 diabetes, insulin injections are necessary to be taken every day. We cannot take insulin as tablets because it is a protein and will be digested in the stomach. To determine the use of insulin patients should use the biosensor which uses a test strip to determine blood glucose concentration. The patient must pierce his/her skin and rub the blood on to the test strip and place it in the biosensor. The device is very accurate and will calculate the exact amount of glucose concentration. This determines whether a patient needs insulin injections or not. A patient will have to test this several times a day. Due to advance in science, a new device is available to help a diabetic (a person with diabetes is called diabetic). This is the insulin pump. It is connected to a very tiny tube called canula which is inserted into the skin to supply insulin. The pump has a monitor on it which calculates blood glucose concentration. The patient can check his glucose concentration and can use the monitor to adjust the amount of insulin entering the blood stream, according to his diet and lifestyle.
Due to advance in science, a new device is available to help a diabetic (a person with diabetes is called diabetic). This is the insulin pump. It is connected to a very tiny tube called canula which is inserted into the skin to supply insulin. The pump has a monitor on it which calculates blood glucose concentration. The patient can check his glucose concentration and can use the monitor to adjust the amount of insulin entering the blood stream, according to his diet and lifestyle.
 reproduce in which results in a higher yield.
reproduce in which results in a higher yield. The single cell proteins are rich in proteins and can be used to replace the use of meat in diet.
The single cell proteins are rich in proteins and can be used to replace the use of meat in diet.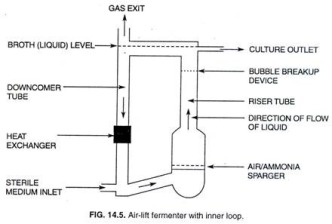 feed humans instead of meat or chicken or even mutton. It has rich amount of proteins and minerals in it. However it is not fermented in a normal bioreactor. A special, long bioreactor is used which forms a loop around itself. This loop fermentor moves the broth around the bioreactor by the help of filtered air compressing into the bioreactor. The material harvested is odorless and colorless and tasteless. Flavorings and colors is added artificially to form its taste exactly like meat.
feed humans instead of meat or chicken or even mutton. It has rich amount of proteins and minerals in it. However it is not fermented in a normal bioreactor. A special, long bioreactor is used which forms a loop around itself. This loop fermentor moves the broth around the bioreactor by the help of filtered air compressing into the bioreactor. The material harvested is odorless and colorless and tasteless. Flavorings and colors is added artificially to form its taste exactly like meat.
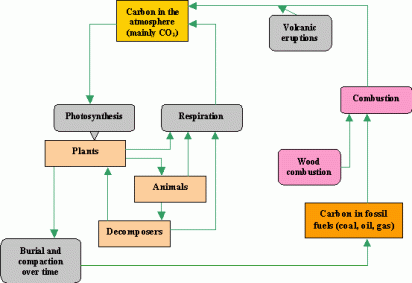 breathe, the carbon dioxide is exhaled and is sent back into the environment. So is the case with microorganisms. The use of fossil fuels have been a disadvantage. The combustion of fossil fuels have added to the carbon cycle. These increase the amount of carbon dioxide in air.
breathe, the carbon dioxide is exhaled and is sent back into the environment. So is the case with microorganisms. The use of fossil fuels have been a disadvantage. The combustion of fossil fuels have added to the carbon cycle. These increase the amount of carbon dioxide in air.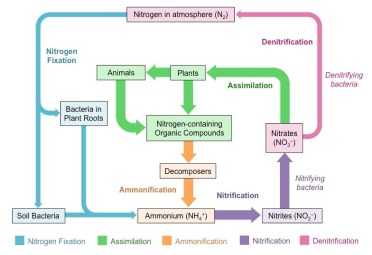 cycle shows that the plants take up the nitrogen from the soil and then is transferred to the herbivore and the consumers along the food chain. When the organisms die they are decomposed by the microorganisms. These microorganisms release the nitrogen in the form ammonia ions. The nitrogen fixing bacteria in the roots of the leguminous plants “fix” the nitrogen in the atmosphere and convert it into ammonium ions which is used by the plants. Other then that the ammonium ions are converted into nitrates by the process of nitrification. This is carried out by the microorganisms called nitrifying bacteria.
cycle shows that the plants take up the nitrogen from the soil and then is transferred to the herbivore and the consumers along the food chain. When the organisms die they are decomposed by the microorganisms. These microorganisms release the nitrogen in the form ammonia ions. The nitrogen fixing bacteria in the roots of the leguminous plants “fix” the nitrogen in the atmosphere and convert it into ammonium ions which is used by the plants. Other then that the ammonium ions are converted into nitrates by the process of nitrification. This is carried out by the microorganisms called nitrifying bacteria.
 had the carrot self pollinated and cross pollinated according to situations. After the new carrot was reproduced it was cross pollinated. This process was applied for quite a lot of time and today we have orange, rich in minerals and tasty sweet carrots. These carrots are called domestic for this reason and are bigger in size and shape compared to the wild carrots. Same is the case with the cabbage family. Many plants which are cabbage shaped like broccoli and cauliflower have been artificially selected from the same plant, Mustard Flower plant.
had the carrot self pollinated and cross pollinated according to situations. After the new carrot was reproduced it was cross pollinated. This process was applied for quite a lot of time and today we have orange, rich in minerals and tasty sweet carrots. These carrots are called domestic for this reason and are bigger in size and shape compared to the wild carrots. Same is the case with the cabbage family. Many plants which are cabbage shaped like broccoli and cauliflower have been artificially selected from the same plant, Mustard Flower plant. common practice. Farmers do this to produce better quality meat and fur and to produce more milk. The farm animals, like a cow and bull, are cross bred and a genetically unique animal is born. That animal is again cross bred to produce another genetically unique animals. This process keeps on happening which is why soon new unique animals are born which produce more milk and better quality fur and meat.
common practice. Farmers do this to produce better quality meat and fur and to produce more milk. The farm animals, like a cow and bull, are cross bred and a genetically unique animal is born. That animal is again cross bred to produce another genetically unique animals. This process keeps on happening which is why soon new unique animals are born which produce more milk and better quality fur and meat.
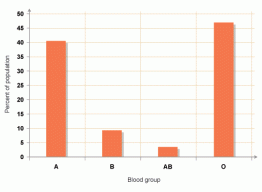 My previous posts have been about discontinuous variation such as blood type, height, flower color, leguminous or non legume plant etc. To make this elaborate, we can take a particular population and set it up in a bar graph and show how the population shows a genetic variation in a particular feature such as blood group.
My previous posts have been about discontinuous variation such as blood type, height, flower color, leguminous or non legume plant etc. To make this elaborate, we can take a particular population and set it up in a bar graph and show how the population shows a genetic variation in a particular feature such as blood group. continuous variation.
continuous variation.
 normal sperm, it results in a zygote having 47 chromosome number rather than 46 (the diploid number of chromosomes in human cells). The offspring born from this is abnormal; it has slow growth rate, facial features indicating abnormality, and low to moderate intelligence. The mutation is common in older woman who fail to produce a normal egg. This means a younger woman has a 1 in 1500 chance of producing an offspring with Down’s Syndrome while in old woman it is 1 in 100 chance that the offspring produced has Down’s Syndrome. The offspring born with Down’s Syndrome may not survive but with proper treatment and medications the child will survive and live for a long lifespan.
normal sperm, it results in a zygote having 47 chromosome number rather than 46 (the diploid number of chromosomes in human cells). The offspring born from this is abnormal; it has slow growth rate, facial features indicating abnormality, and low to moderate intelligence. The mutation is common in older woman who fail to produce a normal egg. This means a younger woman has a 1 in 1500 chance of producing an offspring with Down’s Syndrome while in old woman it is 1 in 100 chance that the offspring produced has Down’s Syndrome. The offspring born with Down’s Syndrome may not survive but with proper treatment and medications the child will survive and live for a long lifespan.
 might not be present. This causes the lack of pigmentation or color in skin, hair and eyes.
might not be present. This causes the lack of pigmentation or color in skin, hair and eyes. turn into sickle shaped. In this state the red blood cells become more fragile and carry less oxygen. The sickle shaped red blood cells are also destroyed by the spleen faster than the normal red blood cells. This causes anemia. Anemia is a disease in which the person is lacking a huge amount of red blood cells.
turn into sickle shaped. In this state the red blood cells become more fragile and carry less oxygen. The sickle shaped red blood cells are also destroyed by the spleen faster than the normal red blood cells. This causes anemia. Anemia is a disease in which the person is lacking a huge amount of red blood cells.
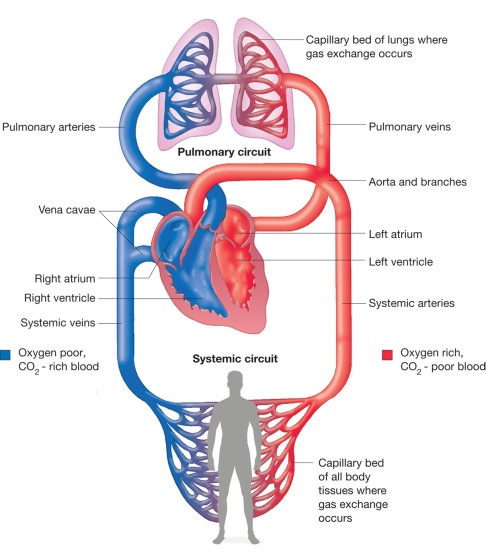 smaller capillaries. The small size of the capillaries help in diffusion of the substances out of the vessels. The blood then travels through the venules and flow to the veins. The veins then return the blood back to the heart. The veins are different than arteries. The veins are a bit flat and have valves inside it which is shaped like half moon which is why it is called the semi lunar valves.
smaller capillaries. The small size of the capillaries help in diffusion of the substances out of the vessels. The blood then travels through the venules and flow to the veins. The veins then return the blood back to the heart. The veins are different than arteries. The veins are a bit flat and have valves inside it which is shaped like half moon which is why it is called the semi lunar valves.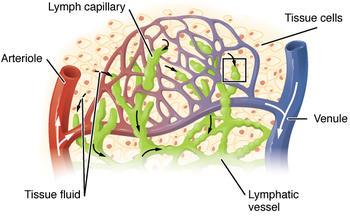 There are another set of tubes called the lymph capillaries which collect the tissue fluid. This fluid is then called lymph. The lymph is then transported to the veins where it diffuses into the blood.
There are another set of tubes called the lymph capillaries which collect the tissue fluid. This fluid is then called lymph. The lymph is then transported to the veins where it diffuses into the blood.
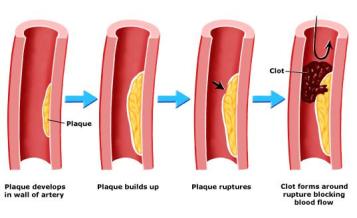 the coronary arteries, they reduce the amount of red blood cells passing through it. This causes the decrease in oxygen supply to the heart. This in turn causes a heart attack.
the coronary arteries, they reduce the amount of red blood cells passing through it. This causes the decrease in oxygen supply to the heart. This in turn causes a heart attack.

 ntigens on the cells of the organs as a foreign cell and produces antibodies against these organs and destroy the organ, this is called organ rejection.
ntigens on the cells of the organs as a foreign cell and produces antibodies against these organs and destroy the organ, this is called organ rejection.